Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite significant advances in the treatment of psoriatic arthritis (PsA), disease control and remission remain a challenge. Research characterizing residual disease burden with current therapies and identifying unmet treatment needs across clinical, patient-focused, and economic domains has been limited. The aim of this review is to establish unmet needs in PsA patients with respect to the clinical, patient-reported and economic outcomes associated with current treatments.
Methods: A review of the literature from January 2008 to May 2019 was conducted. EMBASE and MEDLINE databases were searched to identify full-text, English-language articles and rheumatology conference abstracts evaluating the clinical, patient-reported, and economic outcomes associated with currently available advanced therapies (biologic disease-modifying anti-rheumatic drugs [DMARDs] and targeted synthetic DMARDs) and conventional synthetic DMARDs (csDMARDs) for adults with PsA. Randomized-controlled trials (RCTs), non-interventional observational studies, and economic models evaluating the impact of treatment on at least one outcome from the above categories were eligible for inclusion.
Results: Among 1,026 records initially screened, 100 articles were selected for inclusion. Of these, 52, 74, and 20 studies reported on the impact of treatment on at least one clinical, patient-reported, and economic outcome, respectively. Across studies, treatment with advanced therapies resulted in greater clinical efficacy and effectiveness, improvements in patient-reported outcomes, and higher 12-month persistence rates compared with csDMARD treatment. Advanced therapies were generally found to be cost-effective. Long-term outcomes over four or more years were investigated in 15 studies, revealing improvements in clinical outcomes consistent with shorter-term studies. Clinical and patient-reported outcome data at six and 12 months post-treatment initiation among patients treated with advanced therapies are summarized in Table 1. The proportion of patients achieving minimal disease activity at 12 months post-treatment initiation ranged from 23.6%–64.0%. The impact of advanced therapies on pain and physical functioning varied considerably across RCTs and observational studies. Though commonly assessed in RCTs, little real-world data are available for fatigue, enthesitis, and quality of life in PsA patients initiating advanced therapies.
Conclusion: While more effective than csDMARDs, a substantial proportion of PsA patients still fail to achieve complete disease control or remission with advanced therapies. There remains a need for new treatment options to further improve treatment outcomes in this patient population.
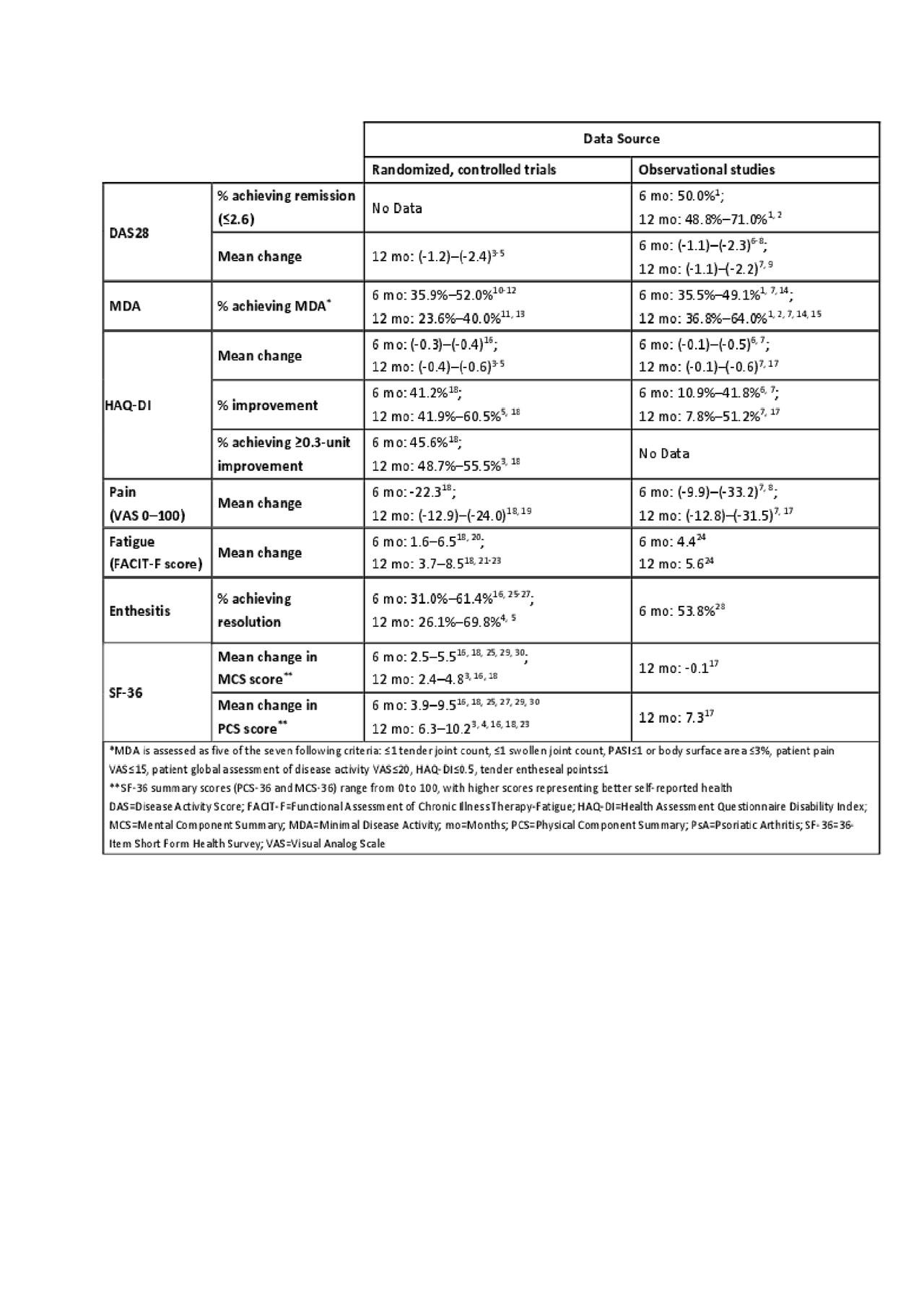
DV56686 PsA_UnmetNeedLitReview_Abstract_FINAL_TABLE

DV56686 PsA_UnmetNeedLitReview_Abstract_FINAL_TABLE2
To cite this abstract in AMA style:
Aletaha D, Georgallis M, Wallace M, Zueger P, Zeidman R. Unmet Treatment Needs in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/unmet-treatment-needs-in-patients-with-psoriatic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/unmet-treatment-needs-in-patients-with-psoriatic-arthritis/
