Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Minimal disease activity (MDA) is associated with reduced radiographic progression and improved quality of life among patients (pts) with PsA.1 Clinical advances made MDA an attainable treatment target, yet it remains challenging for pts to achieve, and fewer achieve very low disease activity (VLDA).1
Understanding the most commonly unmet MDA/VLDA criteria among pts may provide insight into unmet needs with current treatments.
Methods: Eligible pts with PsA in the CorEvitas PsA/SpA Registry (03/2013–10/2023) who initiated biologic/targeted synthetic DMARDs (b/tsDMARDs) had MDA/VLDA computed at treatment initiation (index) and 6 months (range: 5–9 months) post-index. Pts in MDA and VLDA at the index date were excluded from the MDA and VLDA cohorts, respectively, while the VLDA cohort included pts in MDA at index.
At 6 months, pts were classified by number of criteria met out of the 7 MDA/VLDA criteria: achievers (MDA: ≥5/7; VLDA: 7/7); all non-achievers (MDA: ≤4/7; VLDA: ≤6/7); non-achievers by 1 criterion (MDA: 4/7; VLDA: 6/7); and non-achievers by >1 criterion (MDA: ≤3/7; VLDA: ≤5/7). We report the proportion of pts not meeting each MDA/VLDA criterion at index and follow-up.
Results:
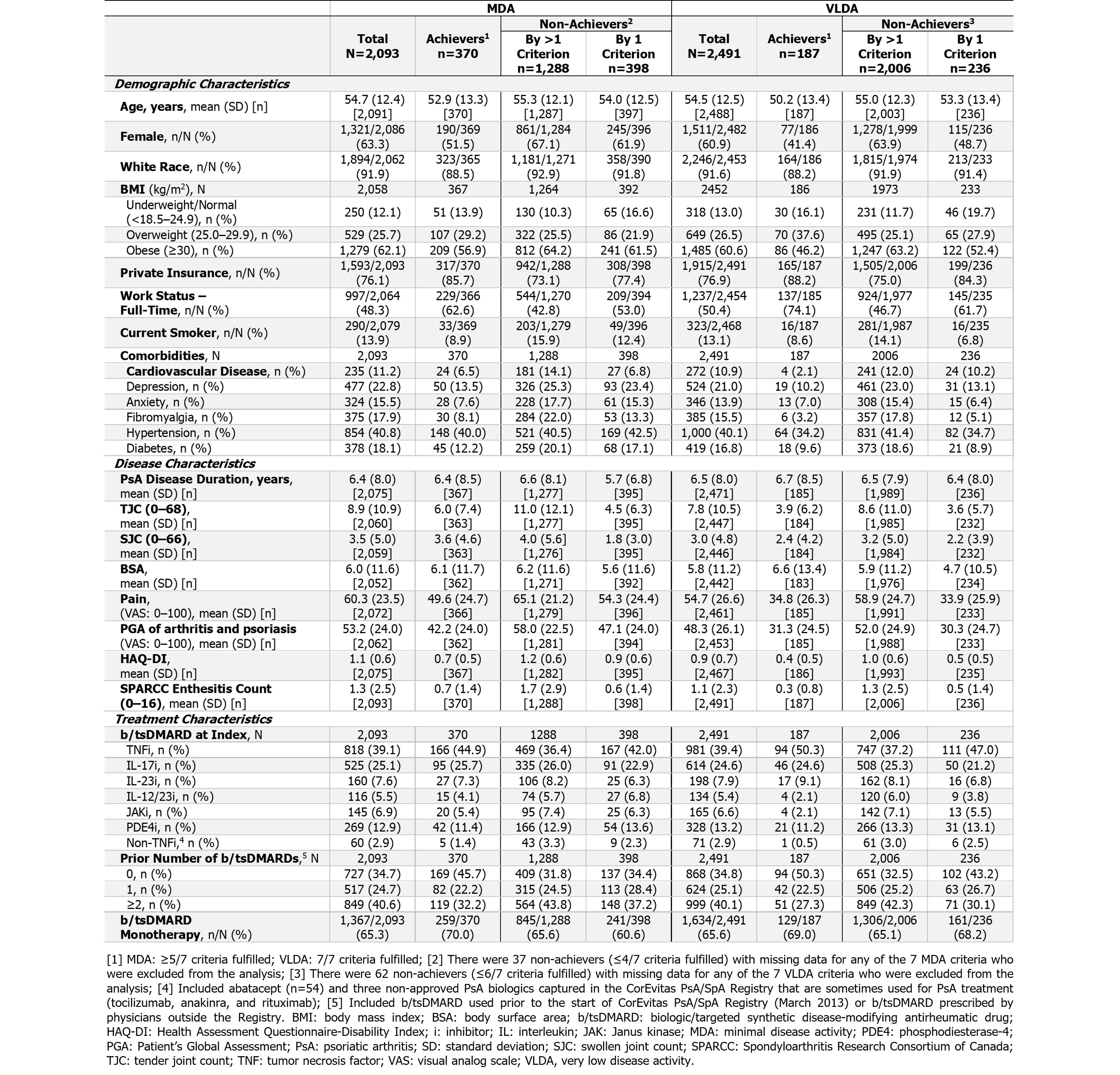
The MDA and VLDA cohorts had 2,093 and 2,491 pts, respectively. Most pts (65%) in both cohorts had prior b/tsDMARD experience at index. Baseline characteristics are summarized in the Table.
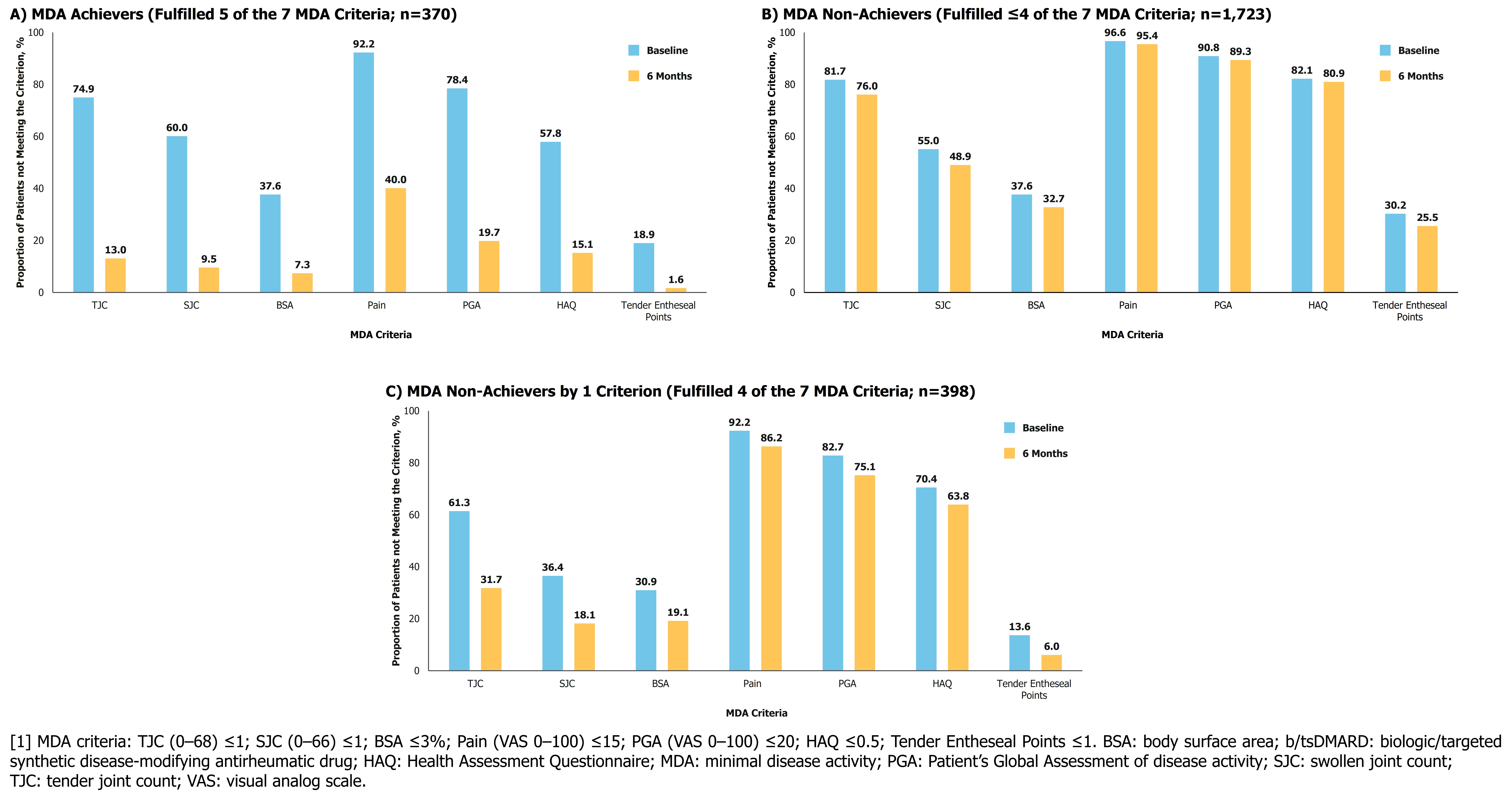
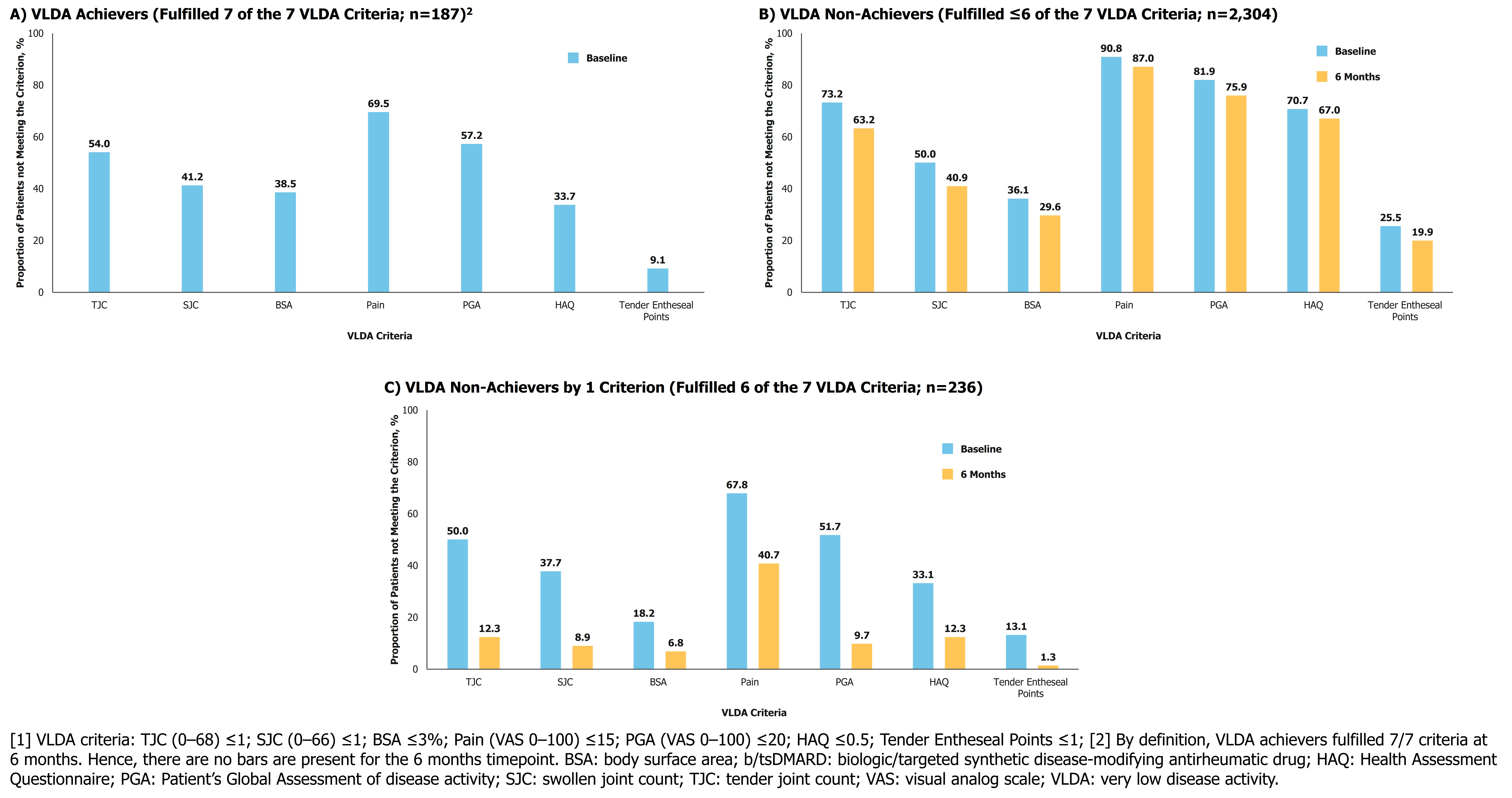
In the MDA cohort, 18% (n=370/2,093) of pts were MDA achievers and 82% (n=1,723/2,093) were non-achievers; 24% (n=398/1,686) of non-achievers (with all criteria available) missed 1 criterion to achieve MDA (Table). In the VLDA cohort, 8% (n=187/2,491) of pts were VLDA achievers and 92% (n=2,304/2,491) were non-achievers; 11% (n=236/2,242) of non‑achievers (with all criteria available) missed 1 criterion to achieve VLDA (Table).
At index, MDA and VLDA non-achievers were older, and a higher proportion were female, current smokers, obese, and had public insurance. They also had higher rates of depression, anxiety, and fibromyalgia, higher disease activity, and worse pt-reported outcomes (PROs) at index (Table).
The proportion of MDA achievers not meeting each criterion reduced drastically after 6 months (Figure 1). However, 40% of pts did not meet the pain threshold at 6 months despite achieving MDA (Figure 1). Among MDA non-achievers, the PRO MDA criterion of pain, Pt’s Global Assessment of disease activity (PGA), and Health Assessment Questionnaire (HAQ) were the least commonly achieved. Among MDA/VLDA non-achievers, these PROs had the least change in the proportion of pts not meeting the MDA/VLDA threshold at 6 months (Figures 1–2).
Among pts who missed 1 criterion to achieve MDA, the most commonly unmet criteria were pain (86%), PGA (75%), and HAQ (64%; Figure 1). Most pts who missed 1 criterion to achieve VLDA did not meet the pain (41%), tender joint count (12%), and HAQ (12%) criteria (Figure 2).
Conclusion: A small proportion of pts achieved MDA after 6 months of b/tsDMARD treatment. Despite achieving MDA, 40% of pts had residual pain. PRO criteria were the least likely to be met among MDA/VLDA non-achievers. A focus on treating pt-reported symptoms may improve achievement of MDA/VLDA.
References:
1. Ortolan A. Semin Arthritis Rheum 2023;62:152237.
To cite this abstract in AMA style:
Ogdie A, Song C, Middaugh N, Marchese M, Eliot M, Low R, Mease P. Unmet Criteria for Achieving Minimal and Very Low Disease Activity Among Patients with Psoriatic Arthritis Initiating Biologic or Targeted Synthetic Disease-Modifying Antirheumatic Drugs in the CorEvitas PsA/SpA Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/unmet-criteria-for-achieving-minimal-and-very-low-disease-activity-among-patients-with-psoriatic-arthritis-initiating-biologic-or-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-the-corevi/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/unmet-criteria-for-achieving-minimal-and-very-low-disease-activity-among-patients-with-psoriatic-arthritis-initiating-biologic-or-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-the-corevi/