Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Absence of joint loading leads to cartilage atrophy and degeneration for unknown reason. We hypothesized that in vivo chondrocytes are exposed to permanent, loading mediated, TGFβ signaling and that loss of loading will result in rapid loss of protective TGFβ signalling. Additionally, we investigated the effects of inflammation on this loading mediated TGFβ signalling.
Methods : Bovine metacarpophalangeal joints were obtained from a local abattoir. TGFβ/Smad2/3P signaling was monitored by immunohistochemistry for phosphorylated-Smad2 (Smad2P) and by gene expression analysis using qPCR. A physiological load of 3 MPa (1 Hz) for 30 min was used for loading experiments. Sulfated glycosaminoglycan (GAG) levels were measured using DMB. Conditioned medium (OAS-CM), obtained from synovial biopsies of OA patients, was used to study inflammation.
Results: Active TGFβ signaling was abundantly present throughout all layers of freshly loaded cartilage. However, after unloading this signalling diminished rapidly and showed significant reduction after 2 hours. Compressive loading of articular cartilage resulted in swift restoration of Smad2P levels as well as potent up-regulation of Smad2/3P dependent gene expression including Tgfb1, and its receptor ALK5. Importantly, this process was repeatable, but could be inhibited by the ALK4/5/7 inhibitor SB-505124, indicating TGFβ or Activin signaling as the driver. However, loading effects could be mimicked by exogenous rhTGFβ1(1 or 10 ng/ml), but not by exogenous rhActivin A (1or 10 ng/ml). Unloading of explants for 2 weeks resulted in profound GAG loss and increased chondrocyte hypertrophy (Col10a1). Remarkably, neither repeated loading nor exogenous TGFβ1 counteracted GAG loss, but repeated loading did inhibit the elevation of Col10a1 expression as effective as exogenous TGF-β. Strikingly, OAS-CM inhibited loading-induced expression of ALK5 and down regulated expression of Tgfbr2, the essential type II receptor for TGFβ signaling, and this was reflected in reduced expression of loading-induced Smad2/3P dependent genes
Conclusion: Our data strongly indicate that, in vivo, articular cartilage has enduring TGFβ signalling when regularly loaded, but which is inhibited in inflammatory conditions. This loading- induced TGFβ signalling has an important role in maintenance of chondrocyte phenotype but not of cartilage GAG content. Based on these results and our earlier findings we suggest that loading-induced TGF-β signalling is crucial to maintain articular cartilage functioning and that this protective system can be impaired under inflammatory conditions. 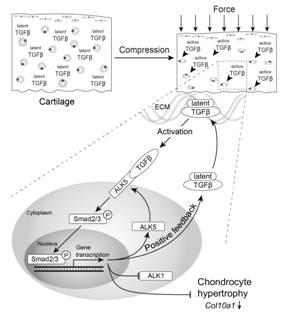 Figure 1: Loading induced Smad2/3P activates a positive feedback loop by inducing expression of its own activating receptor: ALK5, and (inactive) TGFβ1, which after production will bind to the ECM and return the system to its original stage.
Figure 1: Loading induced Smad2/3P activates a positive feedback loop by inducing expression of its own activating receptor: ALK5, and (inactive) TGFβ1, which after production will bind to the ECM and return the system to its original stage.
To cite this abstract in AMA style:
van Caam A, Madej W, Blaney Davidson E, Buma P, van der Kraan PM. Unloading Results in Rapid Loss of TGFβ Signaling in Cartilage: Role of Loading-Induced TGF-Β Signaling in Maintenance of Articular Chondrocyte Phenotype? [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/unloading-results-in-rapid-loss-of-tgf%ce%b2-signaling-in-cartilage-role-of-loading-induced-tgf-%ce%b2-signaling-in-maintenance-of-articular-chondrocyte-phenotype/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/unloading-results-in-rapid-loss-of-tgf%ce%b2-signaling-in-cartilage-role-of-loading-induced-tgf-%ce%b2-signaling-in-maintenance-of-articular-chondrocyte-phenotype/
