Session Information
Date: Monday, November 11, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Treatments, Outcomes, & Measures
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: AWARE (Comparative and Pragmatic Study of Golimumab IV Versus Infliximab in Rheumatoid Arthritis) is an ongoing Phase 4 comparator study designed to provide a real-world assessment of intravenous golimumab (GLM) and intravenous infliximab (IFX) in patients with rheumatoid arthritis (RA). The study recently reached its Primary Endpoint (comparison of the overall incidence of infusion reactions in GLM- vs IFX-treated patients after 52 weeks) with the last patient reaching 52 weeks of treatment or discontinuation from the study. AWARE also records prior use of biologic medications and concomitant use of methotrexate (MTX). Here we report the incidence of infusion reactions among GLM and IFX patients, examining the influence of prior biologic exposure or concurrent use of MTX, reported at baseline.
Methods: AWARE is a prospective, noninterventional, observational, multicenter, 3-year study conducted in the US. RA patients (1,270 adults) were enrolled at the time of initiating treatment with GLM or IFX. All treatment decisions were made at the discretion of the treating rheumatologist. An infusion reaction was any adverse event that occurred during an infusion or within 1 hour after the infusion of either GLM or IFX. Imputations were not performed on these AWARE data. Data shown are mean ± standard deviation.
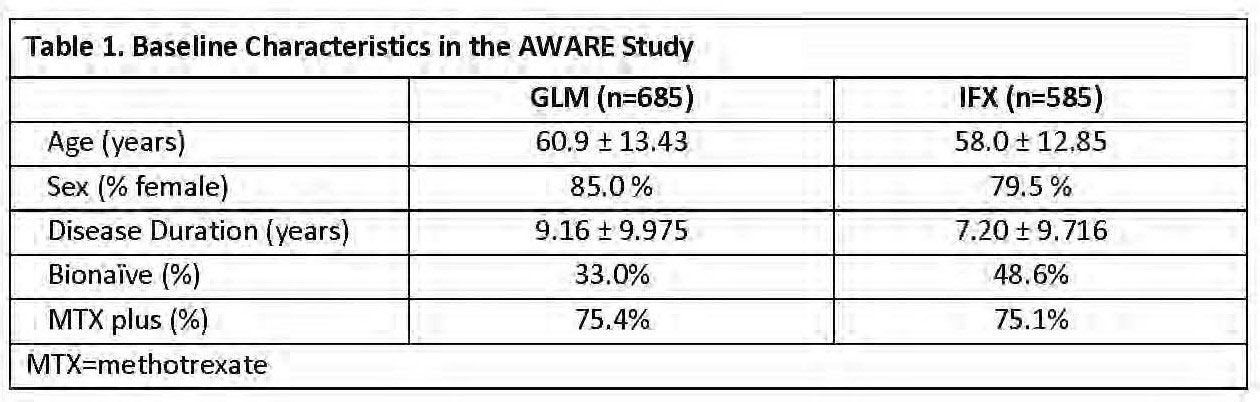
Results: Demographics are shown in Table 1 and the incidence of infusion reactions in the different AWARE cohorts is shown in Table 2. Demographically, GLM and IFX patients were, sex and utilization of MTX at baseline. Both age and disease duration of GLM patients was greater than IFX patients by approximately 2 years. There was a higher proportion of bionaïve patients in the IFX-treated group compared with the GLM-treated group. Overall, infusion reactions occurred more frequently among IFX-treated patients compared with GLM-treated patients. The difference in infusion reaction rates between the IFX- and GLM-treated patients was also evident among subgroups of both bionaïve vs non-bionaïve patients, and among both MTX non-users vs MTX users (characteristics reported at baseline). GLM patients did not report any serious or severe infusion reactions. These were reported rarely (3 of 585 pts) in IFX-treated patients. Among GLM and IFX pts with an infusion reaction, 55.6% of GLM and 77.1% of IFX pts had at least one medication for infusion reaction. Infusion reactions accounted for 9.7% and 35.1% of discontinuations due to adverse events in GLM and IFX pts, respectively.
Conclusion: Whether bionaïve, non-bionaïve, MTX non-user or MTX user at baseline, the incidence of infusion reactions was notably lower among GLM- vs IFX-treated pts. Serious and/or severe infusion reactions did not occur among GLM patients and were rare among IFX patients. IFX patients were more commonly administered mediation for an infusion reaction compared to GLM patients. Infusion reactions accounted for almost four times the number of discontinuations related to adverse events in IFX patients compared to GLM patients.
To cite this abstract in AMA style:
Schwartzman S, Broadwell A, Kivitz A, Black S, Xu S, Kafka S. United States Rheumatology Practice-Based Real-World Evidence of Infusion Reactions in Rheumatoid Arthritis Patients Treated with Intravenous Golimumab or Infliximab: Impact of Prior Biologic Exposure and Methotrexate Utilization [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/united-states-rheumatology-practice-based-real-world-evidence-of-infusion-reactions-in-rheumatoid-arthritis-patients-treated-with-intravenous-golimumab-or-infliximab-impact-of-prior-biologic-exposure/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/united-states-rheumatology-practice-based-real-world-evidence-of-infusion-reactions-in-rheumatoid-arthritis-patients-treated-with-intravenous-golimumab-or-infliximab-impact-of-prior-biologic-exposure/