Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes IV: Outcomes & Comorbidity
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: There is substantial evidence that the brain is targeted early in SLE, likely from disease inception. Data from mouse models and human studies suggest that SLE-related pathogenic mechanisms contribute to brain injury through blood brain barrier disruption, autoantibody and cytokine-mediated injury and microglial synaptic pruning (1). It is unknown whether SLE-related brain abnormalities improve or persist during clinical disease remission. We used rs-fMRI to identify a candidate SLE-related functional network (SLE-Net) and measure individual SLE-Net expression levels in SLE and healthy control (HC) subjects.
Methods: rs-fMRI scans were obtained on 25 SLE and 14 HC enrolled in a prospective observational study. SLE subjects were determined to be in clinical remission (SLE-R; n=9), defined as no disease activity and off all SLE medications except Plaquenil for ≥3 years, or not in remission (SLE-NR).
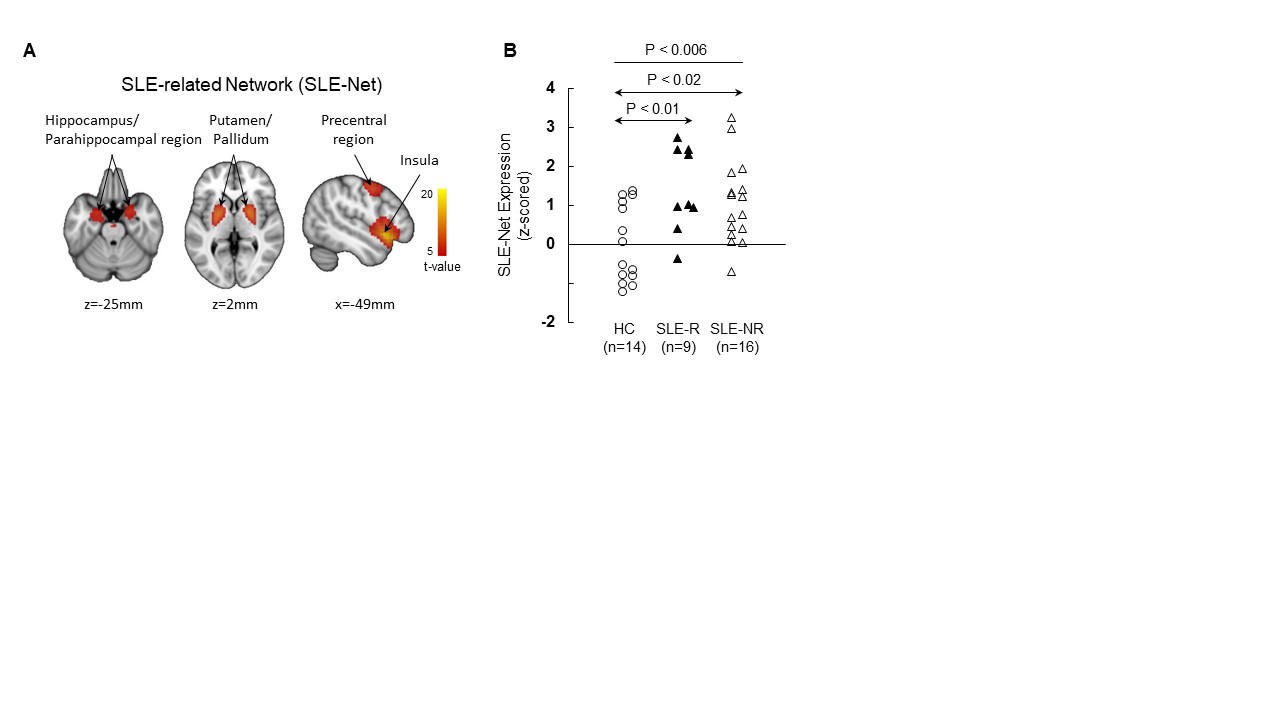
Results: An automated algorithm, blind to group, was applied to rs-fMRI data. For independent components (IC) selection, logistic regression analysis was applied to each of 1,000 bootstrap iterations, revealing three component patterns (IC4 [hippocampus, parahippocampal gyrus, putamen, globus pallidus], IC20 [insula], and IC21 [motor, premotor cortex]), for which the SLE and HC groups differed significantly based on their respective subject scores (p< 0.05). The IC weights (coefficients), 0.35, 0.63, and 0.7, respectively, were determined with a second bootstrap procedure to identify the combination best discriminating SLE from HC. This resulted in a composite SLE-Net, for which the corresponding expression levels accurately discriminated the two groups (P< 0.005; permutation test, 5,000 iterations) (Fig. 1A). Regions contributing to SLE-Net were among the hypermetabolic regions previously identified in SLE with FDG PET (2). SLE-Net expression in individual subjects (Fig. 1B) were elevated in SLE compared to HC (P=0.006). In an exploratory analysis, we used the same approach to identify a candidate SLE-R-specific network (SLE-RNet). Despite the small n (9), a significant SLE-RNet was identified that discriminated SLE-R from HC (p < 0.005; Student t-test). The topography of SLE-RNet was closely related to that of SLE-Net (r=0.93, p< 0.001; voxel-wise correlation with SLE-Net).
Conclusion: In the absence of additional SLE-R subjects for validation, the SLE-NET is considered preliminary. However, our data support the hypothesis that there may be ongoing brain dysfunction despite the appearance of clinical remission and this may impact treatment considerations. 1. N. Kello, E. Anderson, B. Diamond, Cognitive Dysfunction in Systemic Lupus Erythematosus: A Case for Initiating Trials. Arthritis Rheumatol 71, 1413-1425 (2019). 2. M. Mackay et al., Metabolic and microstructural alterations in the SLE brain correlate with cognitive impairment. JCI Insight 4, (2019).
To cite this abstract in AMA style:
MacKay M, vo a, Anderson E, Aranow C, Diamond B, Eidelberg D. Under Assault: Ongoing Brain Dysfunction Identified on Resting State-functional MRI (rs-fMRI) in SLE Patients in Clinical Remission [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/under-assault-ongoing-brain-dysfunction-identified-on-resting-state-functional-mri-rs-fmri-in-sle-patients-in-clinical-remission/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/under-assault-ongoing-brain-dysfunction-identified-on-resting-state-functional-mri-rs-fmri-in-sle-patients-in-clinical-remission/