Session Information
Date: Monday, October 27, 2025
Title: (0934–0954) Systemic Lupus Erythematosus – Animal Models Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Neuropsychiatric SLE (NPSLE) is a severe complication of SLE, characterized by CNS involvement leading to cognitive, behavioral, and neurological deficits. While monoclonal antibodies may offer therapeutic potential for the treatment of SLE, their efficacy in NPSLE is limited by the blood brain barrier (BBB), which restricts drug delivery into the CNS. We evaluated a novel method of focused ultrasound (FUS) with nanobubbles (NB) as a noninvasive strategy to transiently open the BBB and enhance CNS antibody delivery in a NPSLE model.
Methods: We utilized 12-week-old female MRL/lpr mice, an established model of NPSLE. Two separate experiments were conducted to evaluate BBB opening. Firstly, two 10 week-old female B6 mice underwent retro-orbital injection of NB (180 nm diameter, phospholipid shell, octafluoropropane gas core) followed by unilateral transcranial FUS treatment targeting the right hemisphere (NB-FUS), according to a previously established non-neurotoxic protocol from the Ilovitsh laboratory (Gattegno R et al, J Cont Release, 369, 506-516, 2024). FUS parameters included a center frequency of 850 kHz, peak negative pressure of 210 kPa, 4 ms pulses at a repetition frequency of 1 Hz, applied for a total duration of 1 minute. Immediately after FUS application, Evans blue (EB) dye was injected retro-orbitally. Mice were sacrificed, and the brain hemispheres were separated, individually homogenized, and quantitatively analyzed using ELISA to measure the EB concentration and assess differential BBB permeability between the treated and untreated sides.In the second experiment, 6 MRL/lpr lupus-prone and 6 MRL+ control female mice at 10 weeks of age underwent the same NB-FUS protocol. In this study, BBB permeability was assessed by quantitating the fluorescence of two tracers: EB and FITC-labeled dextran (150 kDa), both injected immediately after FUS application. Brains were harvested and processed for fluorescence microscopy to evaluate the intensity of tracer uptake and its distribution in the brain parenchyma.
Results: Quantitative analysis revealed notably higher EB concentrations in the NB-FUS-treated hemispheres in both mice. The following normalized EB concentrations were observed: right hemisphere (1R: 0.353, 2R: 0.239) and left hemisphere (1L: 0.199, 2L: 0.194) (Figure 1), indicating successful spatially targeted disruption of the BBB. In the second experiment, EB penetration was macroscopically visible within the FUS targeted hemisphere in 8/12 mice. Fluorescence microscopy confirmed localized EB and FITC tracer presence in the treated hemisphere with minimal signal in the contralateral side, indicating successful BBB permeabilization. Representative mice are shown in Figure 2. No obvious differences in dye delivery were seen between the two strains. Repeating this experiment with a labeled murine antibody specific for a candidate NPSLE target is in process.
Conclusion: FUS with NB effectively and safely increases BBB permeability in a classic murine NPSLE model. These results support the potential application of this innovative method for targeted CNS delivery of peripherally administered therapeutic agents in autoimmune neuroinflammatory diseases, including NPSLE.
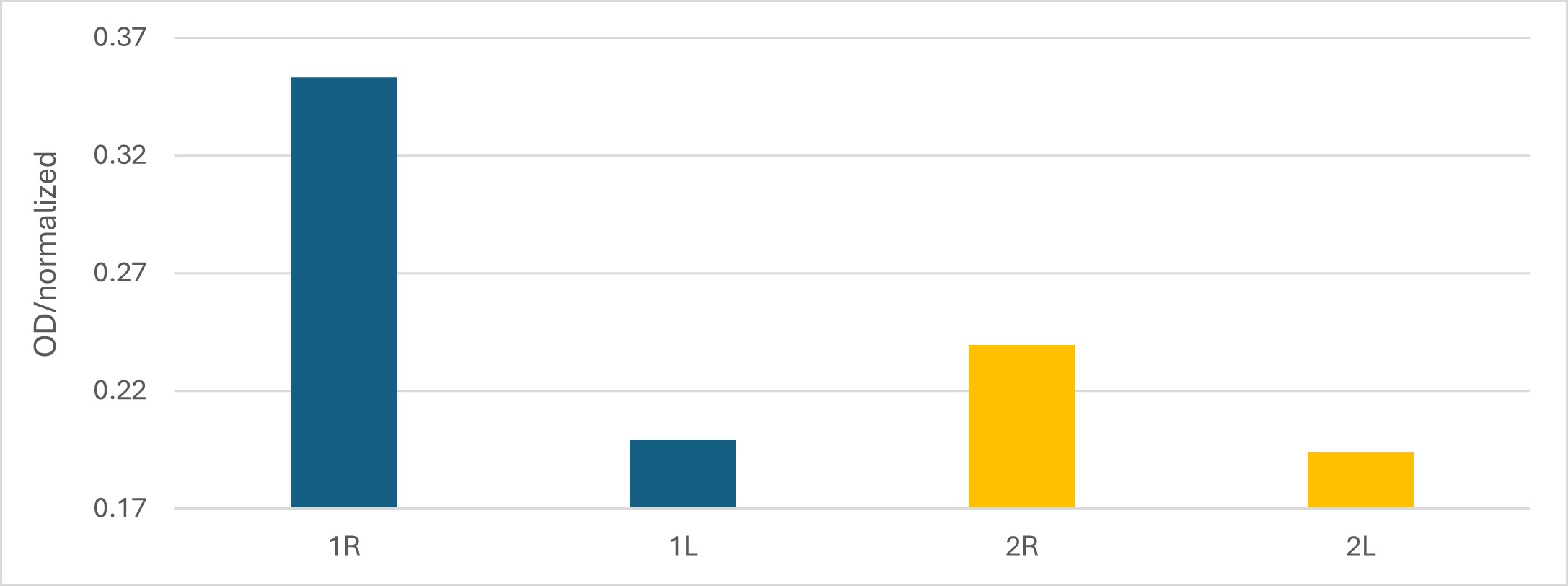 Figure 1. Brain EB concentration. Optical density reading comparison between the FUS-treated right hemispheres (R) and the untreated left hemispheres (L) within individual mice.
Figure 1. Brain EB concentration. Optical density reading comparison between the FUS-treated right hemispheres (R) and the untreated left hemispheres (L) within individual mice.
.jpg) Figure 2: Focal ultrasound application following nanobubble administration locally permeabilizes the BBB and enables delivery of an antibody-sized molecule into the CNS. A. Representative image of MRL/lpr mouse brains immediately after ultrasound treatment, showing localized blue staining indicative of EB penetration into the brain parenchyma. B. A brain section during cryostat slicing, demonstrating widespread EB presence throughout the targeted ultrasound-treated region. C. Magnified (4×) image of the brain highlighting EB distribution in red fluorescence within the treated area. D. Magnified (4×) image showing FITC fluorescence in green (circled in red), indicating distribution of this additional tracer within the treated brain region. E. Merged image of C and D combining EB (red) and FITC (green) signals, demonstrating both tracers’ penetration and co-localization within the ultrasound-targeted area.
Figure 2: Focal ultrasound application following nanobubble administration locally permeabilizes the BBB and enables delivery of an antibody-sized molecule into the CNS. A. Representative image of MRL/lpr mouse brains immediately after ultrasound treatment, showing localized blue staining indicative of EB penetration into the brain parenchyma. B. A brain section during cryostat slicing, demonstrating widespread EB presence throughout the targeted ultrasound-treated region. C. Magnified (4×) image of the brain highlighting EB distribution in red fluorescence within the treated area. D. Magnified (4×) image showing FITC fluorescence in green (circled in red), indicating distribution of this additional tracer within the treated brain region. E. Merged image of C and D combining EB (red) and FITC (green) signals, demonstrating both tracers’ penetration and co-localization within the ultrasound-targeted area.
To cite this abstract in AMA style:
Tehawey D, Gattegno R, Polis B, Bismuth M, Zaknoun M, Ilovitsh T, Putterman C. Ultrasound-Mediated Blood–Brain Barrier Permeabilization Enables Targeted Drug Delivery in a Murine Model of Neuropsychiatric SLE [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/ultrasound-mediated-blood-brain-barrier-permeabilization-enables-targeted-drug-delivery-in-a-murine-model-of-neuropsychiatric-sle/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ultrasound-mediated-blood-brain-barrier-permeabilization-enables-targeted-drug-delivery-in-a-murine-model-of-neuropsychiatric-sle/
