Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Subclinical enthesopathy is recognised in up to 50% of psoriasis patients and is thought to precede inflammatory PsA. It is not known if effective treatment in the asymptomatic phase can reduce inflammation and damage. Studies have assessed enthesitis only as a secondary endpoint in established PsA. This study used ultrasound to investigate the effect of ustekinumab on subclinical enthesopathy in asymptomatic treatment-naive patients with psoriasis.
Methods: 73 new patients with severe psoriasis (PASI>10) and no PsA (absence of inflammatory arthritis to satisfy the CASPAR criteria) were screened using ultrasound. Exclusion criteria included rheumatological disease, RF or ACPA positivity and prior treatment with DMARDs. Patients were eligible if they exhibited inflammatory sonographic changes that fulfilled the OMERACT definition of enthesopathy in at least one peripheral enthesis and met the screening requirements for biologic therapy. 23 psoriasis patients received ustekinumab at week 0, 4, 16, 28 and 40 and underwent further sonography of 26 entheses at week 0, 12, 24 and 52. The same protocol was used once only in 23 healthy controls for comparison. The primary endpoint was the change in sonographic enthesopathy inflammation score (extrapolated from the GUESS score) at 24 weeks. This was a prospective proof-of-concept study (no power calculation). Analysis was primarily descriptive with effect size (Cohens d) and paired t-tests provided for enthesopathy scores.
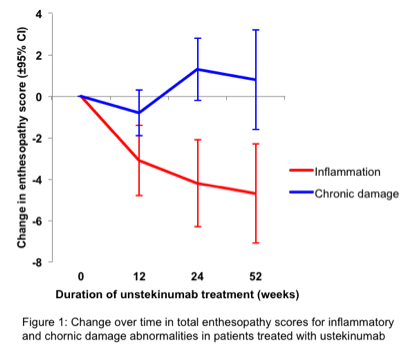
Results: 24% of 598 entheses in patients at week 0 (median (IQR) 6 (4,9) out of 26 per patient) had at least one inflammatory entheseal abnormality (thickening, hypoechogenicity, PD signal) compared to 5% in controls, reducing to 14% and 10% after 24 and 52 weeks of treatment respectively (fig. 1). Mean inflammation scores reduced by 42% to week 24 (mean (95% C.I.) reduction -4.2(-6.3,-2.1) d=1.2 p<0.001) and by 47.5% (-4.7 (-7.1,-2.3) d=1.3 p=0.001) to week 52. Of 187 inflammatory abnormalities found at week 0, 116 (62%) resolved, 4 (7%) improved, 55 (29%) remained unchanged and 2 (1.1%) worsened by week 24. 38 new abnormalities developed on treatment. There were no differences in scores between those achieving PASI90 (n=17) and those not at week 24 (difference -0.3 95% C.I.-4.5,3.8). Chronic damage abnormalities (calcification, enthesophytes, erosions, bone cortex irregularities) did not improve with therapy. 16% entheses at week 0 had at least one abnormality compared to 6% in controls, increasing to 19% and 20% after 24 and 52 weeks of therapy respectively (fig. 1). Mean chronicity scores increased by 17% to week 24 (1.3 (-0.2,2.7) d=0.5 p<0.082), and by 10% (0.8 (-1.6,3.1) d=0.2 p=0.512) to week 52.
Conclusion: This study suggests that ustekinumab therapy for psoriasis suppresses the inflammatory features of subclinical enthesopathy supporting the concept that early intervention may prevent arthritis evolution.
To cite this abstract in AMA style:
Savage L, Goodfield M, Hensor EMA, Emery P, McGonagle D. Ultrasonographic Improvement of Peripheral Subclinical Enthesopathy in Therapy-Naive Patients Treated with Ustekinumab for Chronic Plaque Psoriasis: A 52-Week, Prospective, Open Label, Controlled Cohort Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/ultrasonographic-improvement-of-peripheral-subclinical-enthesopathy-in-therapy-naive-patients-treated-with-ustekinumab-for-chronic-plaque-psoriasis-a-52-week-prospective-open-label-controlled-coho/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ultrasonographic-improvement-of-peripheral-subclinical-enthesopathy-in-therapy-naive-patients-treated-with-ustekinumab-for-chronic-plaque-psoriasis-a-52-week-prospective-open-label-controlled-coho/