Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Type I interferons (IFN) are pivotal in the pathogenesis of SLE, with studies showing high IFN gene signature (IGS) status associated with certain organ manifestations, autoantibody profiles, and disease severity. With novel medications targeting the interferon pathway, an enhanced understanding the IGS in patients with SLE is necessary. Recent studies have suggested that IFN levels remain stable overtime despite changes in disease activity and treatment. In this study, we investigated the differences between IGS levels in relation to clinical characteristics in a large cohort of patients with patients with SLE.
Methods: Patients who met 2019 EULAR/ACR classification criteria for SLE, from a single center, were included. Whole blood collected from a single time point was analyzed for IGS by the DxTerity assay, categorizing patients into IFN high or IFN low status. SLEDAI-2K organ domains were cumulatively characterized from 5 years prior to whole blood collection to last available visit. Clinical characteristics, including SLICC/ACR damage index (SDI), cumulative antibody status, glucocorticoid and immunosuppressive use, were analyzed according to IFN status.
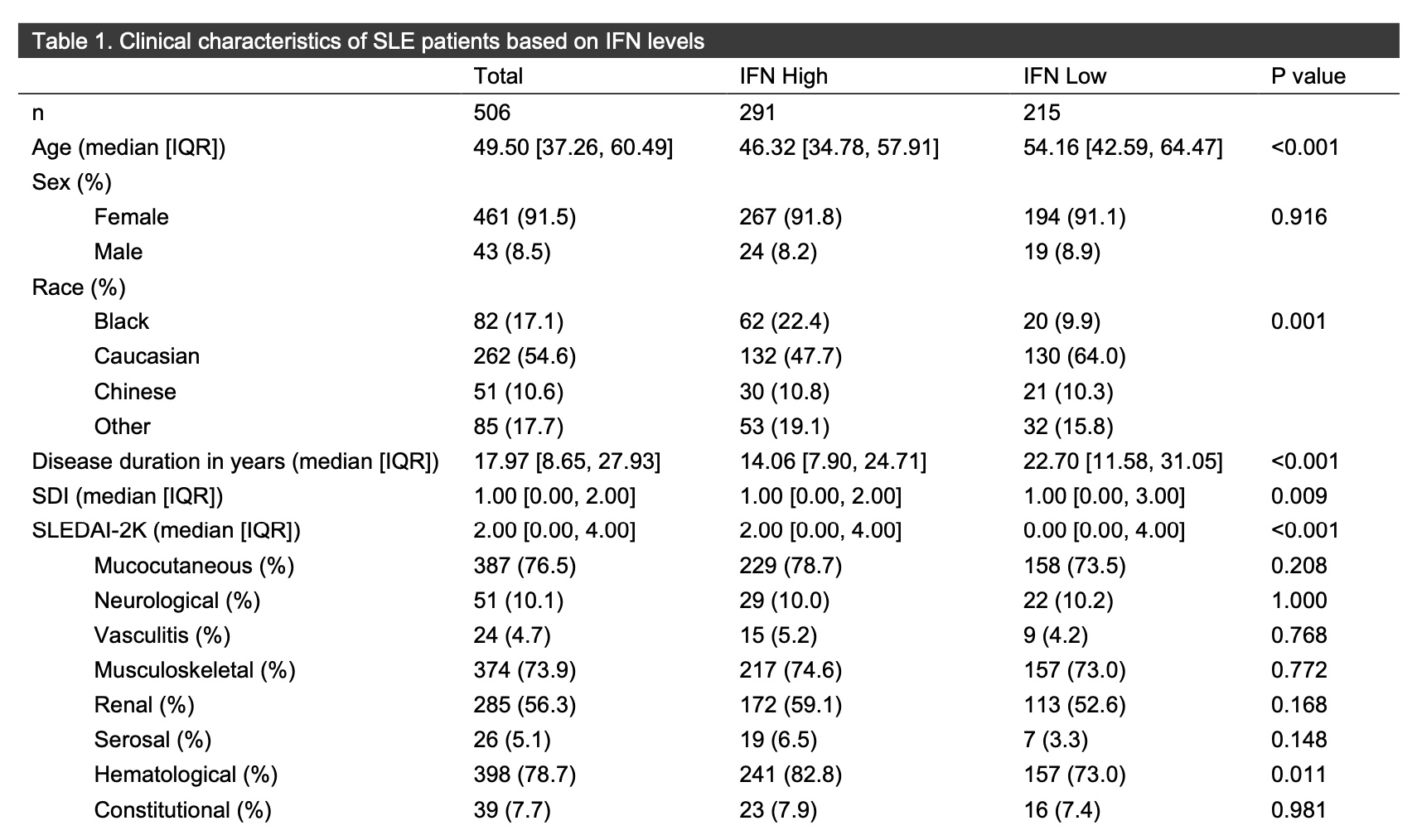
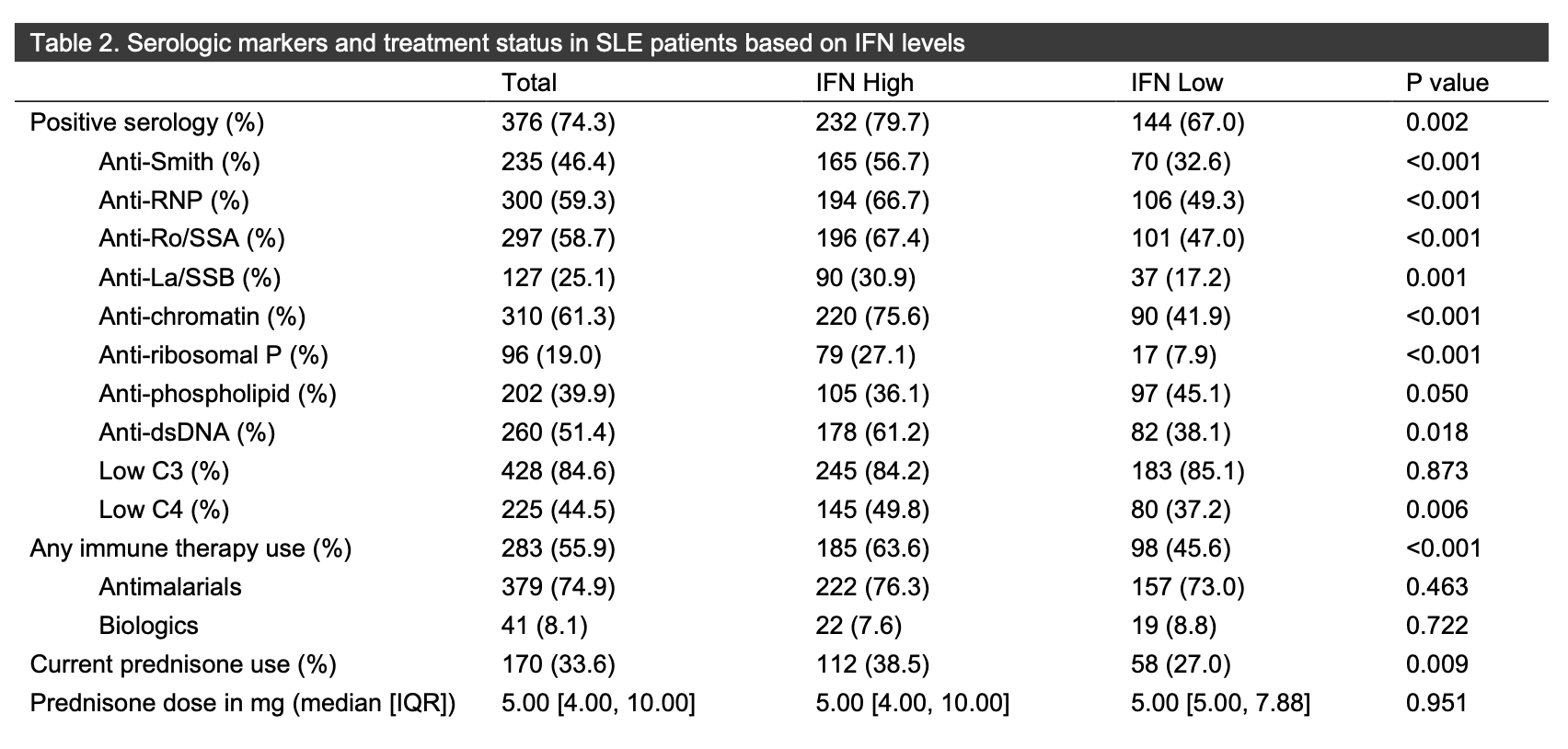
Results: In total, 506 patients with median age of 49.53 years (IQR 37.27-60.54) were included, with 291 (57.5%) IFN high and 215 (42.5%) IFN low. More Black patients were IFN high (75.6%), while more Caucasian patients (49.6%) were IFN low. The median duration of SLE disease was longer in the IFN low group (22.7 years [IQR 11.58-21.05]) than in the IFN high group (14.06 [QR 7.90-24.71]) (p< 0.001). There was no difference in the proportion of the individual SLEDAI-2K organ domains between the IFN high and low groups, aside from presence of the hematologic domain (IFN high 82.8% versus 73.0% [p=0.011]). The median SLEDAI-2K score was higher in the IFN high group (2.00 [IQR 0.00-4.00]) then in the IFN low group (0.00 [IQR 0.00-4.00]) (< 0.001). The SDI was similar between the two groups. More patients in the IFN high vs. low group had positive Smith (56.7% vs. 32.6% [p< 0.001]), RNP (66.7% vs. 49.3% [p< 0.001]), Ro (67.4% vs. 47.0% [p< 0.001]), La (30.9% vs. 17.2% [p=0.001]), chromatin (75.6% vs. 41.9% [p< 0.001]), dsDNA (61.2% vs. 38.1% [p< 0.001]) and ribosomal P (27.1% vs. 7.9% [p< 0.001]) autoantibodies, with no differences in levels of C3 and C4 or in the presence of antiphospholipid antibodies. Numerically more patients with IFN high status were on glucocorticoids (112; 38.5%) then were patients with IFN low status (58, 27%) (p=0.009). More patients with IFN high status were on immunomodulatory agents (185; 63.6%) vs. the IFN low group (98; 45.6%) (p< 0.001), though there was no difference in use of antimalarials or biologics.
Conclusion: In this large cohort of patients with SLE, IGS status may help to predict disease severity, use of glucocorticoids, and overall use of immunomodulatory therapy, though IFN level did not predict presence of SLEDAI-2K organ domains. More studies are needed to assess those clinical characteristics associated with an elevated IGS and to determined who may respond best to type I IFN targeted therapies.
To cite this abstract in AMA style:
Smith J, Whittall Garcia L, Bonilla D, Li Q, Terbrueggen R, Wijesuriya H, Richards I, Jacobs A, Wither J, Gladman D, Touma Z. Type I Interferon Status and Clinical Manifestations in a Large Cohort of Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/type-i-interferon-status-and-clinical-manifestations-in-a-large-cohort-of-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/type-i-interferon-status-and-clinical-manifestations-in-a-large-cohort-of-patients-with-systemic-lupus-erythematosus/