Session Information
Date: Sunday, November 12, 2023
Title: (0543–0581) SLE – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Aberrant activation of type I IFN, and subsequent adverse clinical implications, have been widely discussed in relation to SLE. With the lack of consistent data to support an association between disease activity and type I interferon, we hereby explore an alternative role of type I interferon in SLE pathogenesis. Continuous exposure to type I IFN is implicated in regulating immune checkpoint genes in vitro and in the context of a viral infection. Based on these findings, we investigated whether IFN exposure also regulates immune checkpoint genes in SLE, using bulk RNA-seq analysis.
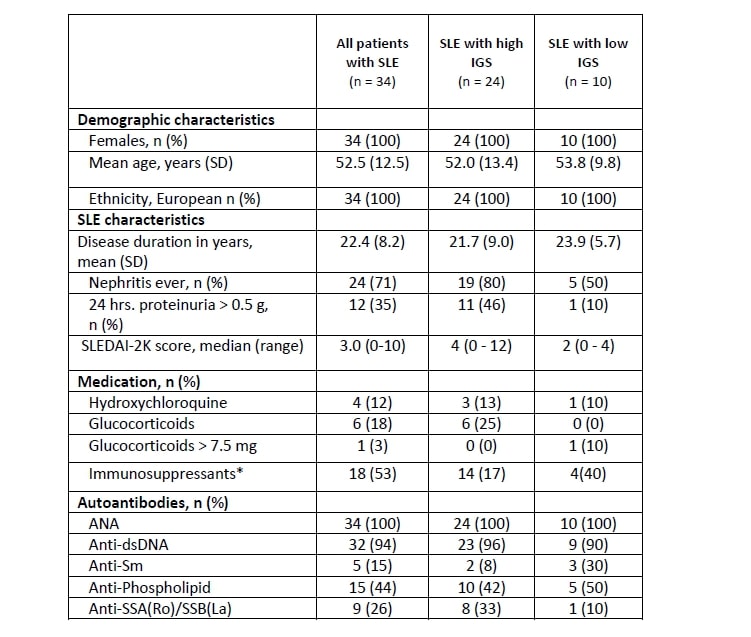
Methods: We measured whole blood RNA-seq in 34 patients fulfilling the 2019 EULAR/ACR classification criteria for SLE and 15 age- and gender-matched healthy controls (HC). SLE disease activity was determined according to the SLE Disease Activity Index 2000 (SLEDAI-2K) score1. Whole blood samples were collected in PAXgene blood tubes (Qiagen) and analyzed for mRNA expression levels of 24 immune checkpoint genes and 12 type I IFN-stimulated genes using NanoString nCounter Technology. All expressions levels were represented as log2 fold change relative to HC. A compound type I IFN gene expression score (IGS) was calculated based on the median log2 fold change of type I IFN-stimulated gene expression and patients were grouped according to low or high IGS (IGS >1). Statistical analyses were performed using R version 3.6.1. and SPSS version 25.
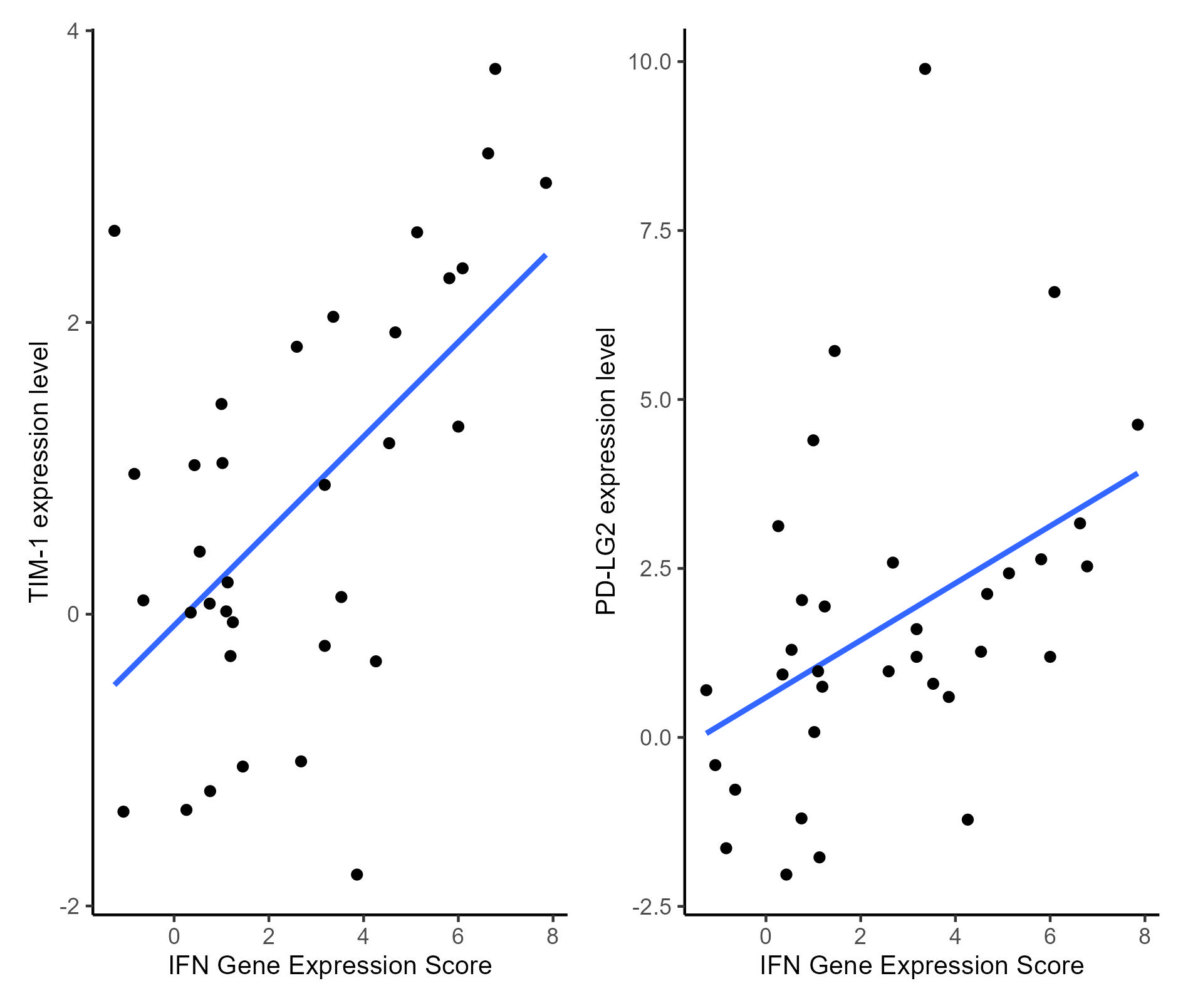
Results: Demographic and clinical characteristics of SLE patients are detailed in Table 1. Twenty-four SLE patients (70%) had a high IGS. We found that genes coding for the immune checkpoints TIM-3 and PD-LG2 were upregulated in SLE patients with a high IFN score compared with HC; log2 fold changes were 0.36 and 0.89 and P-values were 0.0046, 0.0042 for TIM-3 and PD-LG2 respectively. Expression of the immune checkpoint genes paralleled the expression of the IGS but only for those patients who had a high expression of type I IFN stimulated genes (Figure 1). This was confirmed by a generalized linear model analysis showing a significant positive association between the IGS and expression of TIM-3 and PD-LG2 in SLE patients (P= 0.0001 and 0.006, respectively).
The median (range) SLEDAI-2K scores were 4.0 (0–10) and 2.0 (0–5) for the high and low IGS SLE patients, respectively. A generalized linear model analysis showed that SLEDAI-2K scores did not associate with the expression of TIM-3 and PD-LG2, arguing against the notion that differential expression of immune checkpoint genes in high IGS SLE patients may be related to disease activity.
Conclusion: This study revealed differential mRNA expression of immune checkpoint genes TIM-1 and PD-LG2 in a type I IFN-associated manner, which did not associate with disease activity. These results are in line with previous observations that type I IFN activity does not necessarily reflect SLE disease activity. With this, we suggest that persistent type I IFN activation perhaps regulates inhibition of immune cells in SLE patients and other rheumatic diseases characterized by such type I IFN activation.
SLEDAI_2K: SLE Disease Activity Index 2000; ANA: anti-nuclear antibodies; dsDNA: double-stranded DNA; Sm: Smith.
*: Azathioprine, mycophenolate mofetil, cyclophosphamide, rituximab, and/or methotrexate.
To cite this abstract in AMA style:
Siddiqi K, Zinglersen A, Iversen K, Jacobsen S. Type I IFN-associated Regulation of Immune Checkpoint Genes in Patients with SLE [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/type-i-ifn-associated-regulation-of-immune-checkpoint-genes-in-patients-with-sle/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/type-i-ifn-associated-regulation-of-immune-checkpoint-genes-in-patients-with-sle/