Session Information
Date: Friday, November 6, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Clinical Manifestations
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Management of Type 2 SLE (widespread pain, fatigue, depression, sleep distrubance, and cognitive dysfunction) is challenging and often requires multiple medications to ameliorate symptoms. Polypharmacy can increase the risk of drug interactions, side effects, adverse reactions, non-adherence, and lead to greater healthcare costs. Many medications used for Type 2 SLE are associated with QTc interval prolongation. The purpose of this study was to examine symptoms and Type 2 medication polypharmacy.
Methods: This was a cross-sectional study of SLE patients (SLICC 2012 criteria) enrolled in a university registry from May 2018-March 2019. All patients completed the Systemic Lupus Activity Questionnaire (SLAQ), Patient Health Questionnaire-9 (PHQ-9), and 2016 ACR Fibromyalgia criteria questionnaires. Type 2 SLE was defined as a polysymptomatic distress (PSD) score ≥8. Type 2 medications included antidepressants (serotonin and norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), mirtazapine, bupropion, muscle relaxers (cyclobenzaprine, baclofen, metaxalone), gabapentinoids (gabapentin, pregabalin), topiramate, zolpidem, trazodone, tramadol, anxiolytics, stimulants, and DHEA. Relationships between clinical variables and medications in different groups were analyzed using ANOVA and Fisher’s exact test.
Results: One hundred and fifty-nine patients completed surveys (96% female, 66% Black, 42% Type 2 SLE.) Type 2 medications were prescribed in 50% of patients. Patients on Type 2 medications were older and had longer duration of SLE (Table 1). Multiple Type 2 medication use was more frequent in Type 2 SLE patients than non-Type 2 patients (43% vs 25.7%). The average number of Type 2 medications was 1.9. Of patients taking Type 2 medications, 72% were taking at least one QTc interval prolonging Type 2 medications (mean 1.3 medications).
Despite pharmacologic therapy, 58% of SLE patients had active Type 2 SLE (Table 2). The number of patients with depression and Type 2 SLE activity was greater in those on multiple medications. Moreover, the severity of nearly all Type 2 SLE symptoms including widespread pain, fatigue, depression, forgetfulness and sleep dysfunction was greatest in those on more than one Type 2 medication compared to no Type 2 medications or one Type 2 medication.
Conclusion: SLE patients have distressing Type 2 symptoms that often require complex pharmacologic interventions. Although the majority of patients with Type 2 SLE activity take therapies aimed at these symptoms, they continue to have a multitude of symptoms. Furthermore, severe symptoms persist despite nearly half of patients taking multiple medications. Several Type 2 medications can prolong the QTc interval, which when combined with other QTc prolonging medications such as hydroxychloroquine, can augment the risk of arrhythmia. The average number of Type 2 SLE medications is similar to the number used for Type 1 SLE, highlighting the difficulty managing Type 2 SLE, the need for targeted Type 2 therapeutics, and the importance of non-pharmacologic management.
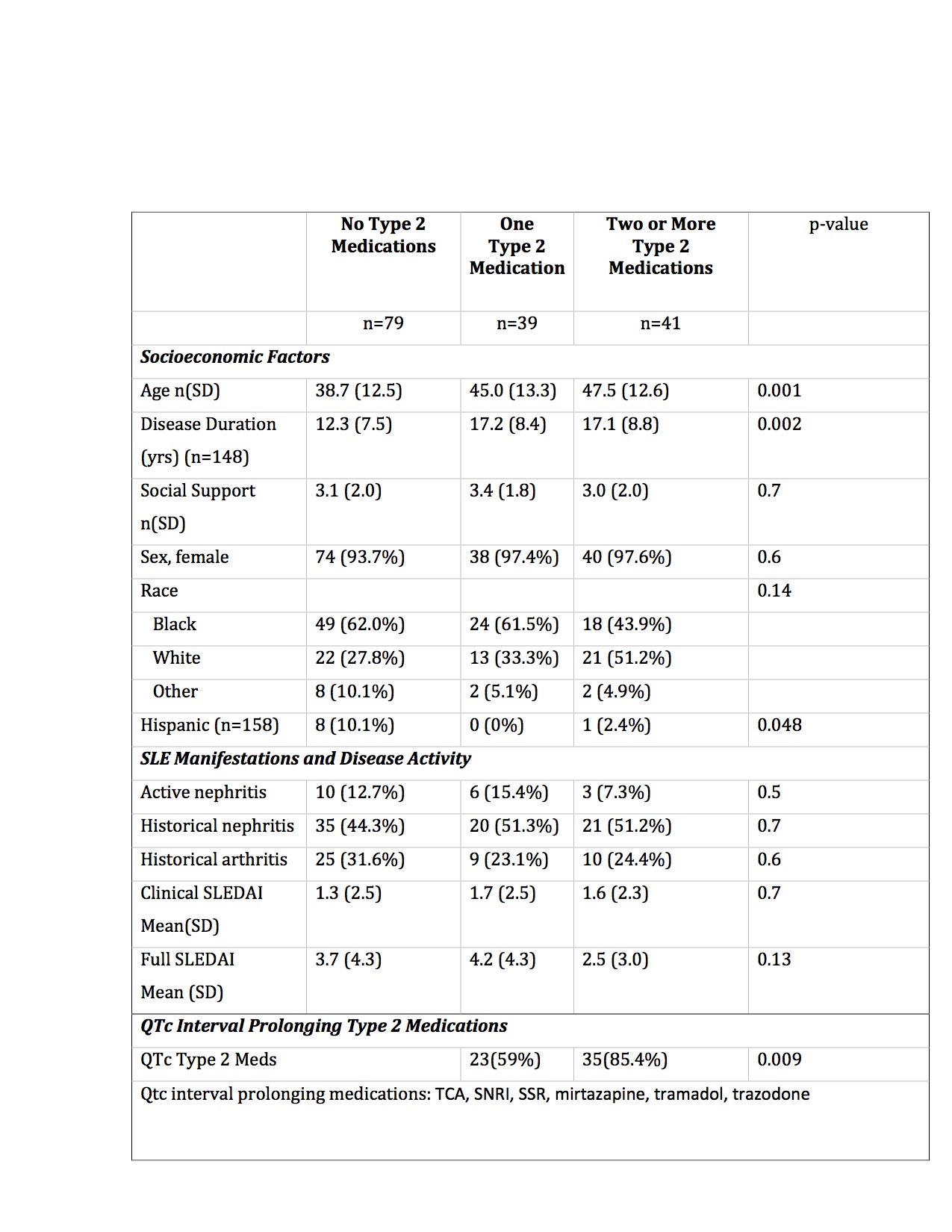 Table 1. Characteristics of SLE patients by Type 2 medication use
Table 1. Characteristics of SLE patients by Type 2 medication use
 Table 2. Type 2 SLE symptoms and Type 2 medication polypharmacy
Table 2. Type 2 SLE symptoms and Type 2 medication polypharmacy
To cite this abstract in AMA style:
Whitney R, Eudy A, Coffman C, Clowse M, Criscione-Schreiber L, Doss J, Sadun R, Sun K, Rogers J. Type 2 SLE Symptoms Persist Despite Type 2 Medication Polypharmacy [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/type-2-sle-symptoms-persist-despite-type-2-medication-polypharmacy/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/type-2-sle-symptoms-persist-despite-type-2-medication-polypharmacy/
