Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The benefits of tapering are a decreased risk of long-term adverse events and a reduction of health care costs, especially when bDMARDs are tapered. However, tapering treatment may lead to more transient or persistent disease flares, which have a direct impact on patients’ lives and societal costs. We aimed to evaluate the two year cost-utility ratio between tapering the csDMARD first followed by the TNF-inhibitor, and tapering the TNF-inhibitor first followed by the csDMARD.
Methods: The TARA trial is a multicenter single-blinded randomized controlled trial. RA patients that used a csDMARD(s) plus a TNF-inhibitor and who had a well-controlled disease for at least 3 months, defined as a DAS≤2.4 and a swollen joint count (SJC) ≤1, were included. Patients were randomized into gradual tapering their csDMARD in the first year followed by the TNF-inhibitor in the second year, or vice versa. Medication was tapered in three steps over the course of 6 months. Gradual tapering was done by cutting the dosage into half, a quarter and thereafter it was stopped. Data on quality adjusted life years (QALYs, measured with the Dutch EuroQol [EQ5D]), health care costs and productivity costs were used to calculate the Incremental Cost Effectiveness Ratio (ICER). The ICER, the cost-effectiveness acceptability curve (CEAC), and the incremental net monetary benefit (iNMB) were used to assess cost-effectiveness between both tapering strategies.
Results: Of the 189 included patients, 94 started tapering their TNF-inhibitor first, while the other 95 tapered their csDMARD first. QALYs (sd) were, respectively, 1.64 (0.22) and 1.65 (0.22). Medication costs were significantly lower in the patients who tapered the TNF-inhibitor first, while indirect cost were higher due to more productivity loss (p=0.10). Therefore, total costs (sd) were €38,833 (€39,616) for tapering csDMARDs first, and €39,442 (€47,271) for tapering the TNF-inhibitor first (p=0.88). The ICER (95% CI) between tapering csDMARDs first minus the TNF-inhibitor first was €60,919 per QALY (95% CI, -€90,638 per QALY to €212,475 per QALY)(figure 1). The iNMB was €1134 (95% CI €761 to €1507) in favor of tapering TNF-inhibitor first for a willingness-to-pay (WTP) level of €50,000, which is the current level of WTP in the Netherlands for treatment of RA (figure 1). According to the CEAC, for WTP levels < €53,800 tapering the csDMARD first has the highest probability of being cost-effective, while for WTP levels >€83,800 tapering the TNF-inhibitor first has the highest probability (figure 2).
Conclusion: Our economic evaluation shows that costs are similar for both tapering strategies. Depending on the WTP threshold, either tapering the TNF-inhibitor first or csDMARD first has the highest probability of being cost-effective.
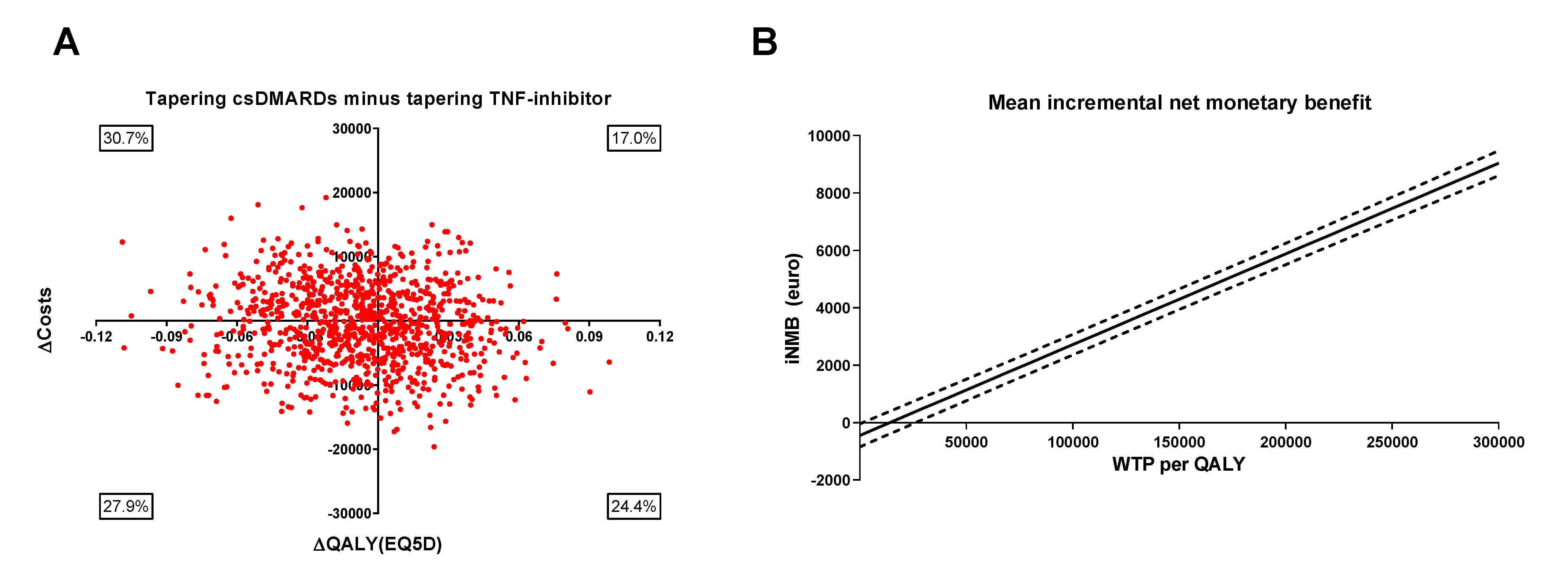 Figure 1 Summary of economic evaluation of tapering csDMARDs first minus tapering TNF-inhibitor first. (A) Results of 1000 bootstrapped replications, presented in a cost-effectiveness plane which represents uncertainty of the cost-effectiveness ratio. (B) Mean incremental net monetary benefit (iNMB) for tapering csDMARDs minus tapering TNF-inhibitors with 95% confidence intervals plotted against different levels of willingness to pay (WTP) per quality adjusted life year (QALY). The iNMB was calculated as the incremental benefit times different levels of WTP, minus the incremental costs. csDMARDs: conventional synthetic DMARDs; iNMB: incremental net monetary benefit; QALY: quality adjusted life year; WTP: willingness to pay.
Figure 1 Summary of economic evaluation of tapering csDMARDs first minus tapering TNF-inhibitor first. (A) Results of 1000 bootstrapped replications, presented in a cost-effectiveness plane which represents uncertainty of the cost-effectiveness ratio. (B) Mean incremental net monetary benefit (iNMB) for tapering csDMARDs minus tapering TNF-inhibitors with 95% confidence intervals plotted against different levels of willingness to pay (WTP) per quality adjusted life year (QALY). The iNMB was calculated as the incremental benefit times different levels of WTP, minus the incremental costs. csDMARDs: conventional synthetic DMARDs; iNMB: incremental net monetary benefit; QALY: quality adjusted life year; WTP: willingness to pay.
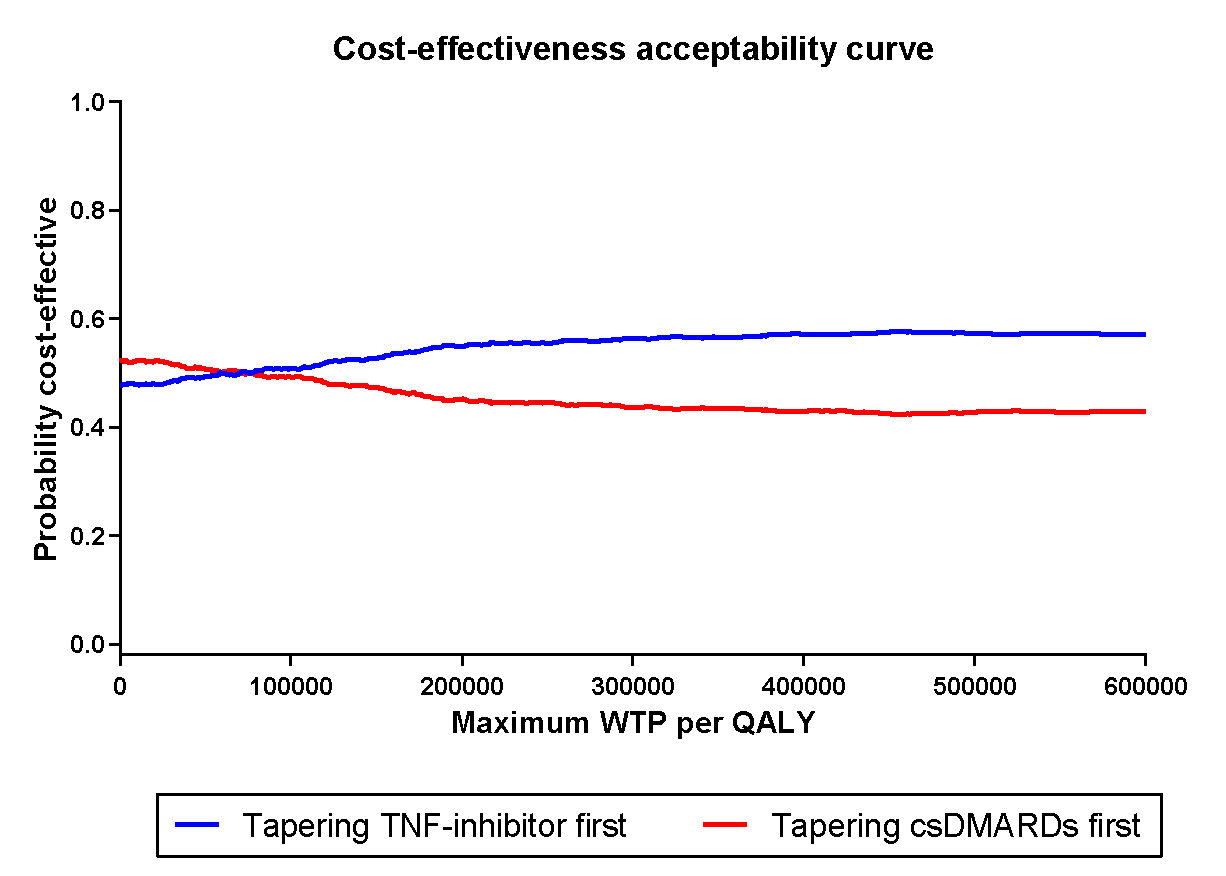 Figure 2 Cost effectiveness acceptability curve for tapering csDMARDs first versus tapering TNF-inhibitor first. Results of 1000 bootstrapped replication, presented for several levels of willingness to pay, indicated per quality adjusted life year (QALY). csDMARDs: conventional synthetic DMARDs; QALY: quality adjusted life year; WTP: willingness to pay.
Figure 2 Cost effectiveness acceptability curve for tapering csDMARDs first versus tapering TNF-inhibitor first. Results of 1000 bootstrapped replication, presented for several levels of willingness to pay, indicated per quality adjusted life year (QALY). csDMARDs: conventional synthetic DMARDs; QALY: quality adjusted life year; WTP: willingness to pay.
To cite this abstract in AMA style:
van Mulligen E, Weel A, Hazes M, van der Helm - van Mil A, de Jong P. Two-year Cost-effectiveness Between Two Gradual Tapering Strategies in Rheumatoid Arthritis: Cost-utility Analysis of the TARA Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/two-year-cost-effectiveness-between-two-gradual-tapering-strategies-in-rheumatoid-arthritis-cost-utility-analysis-of-the-tara-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-year-cost-effectiveness-between-two-gradual-tapering-strategies-in-rheumatoid-arthritis-cost-utility-analysis-of-the-tara-trial/
