Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Immunosuppressive treatments inhibit vaccine-induced immunity. We evaluated if a two-week interruption of methotrexate treatment immediately after COVID-19 booster improved antibody response against spike protein of the receptor binding domain (S1-RBD) and live virus neutralization (ancestral Wuhan and Omicron BA.1) in patients with immune mediated inflammatory diseases (IMIDs).
Methods: We conducted an open-label, prospective, parallel-group, randomized controlled, superiority trial in 26 UK hospitals. Adults attending Rheumatology and Dermatology clinics taking methotrexate (≤25mg/week) for ≥3 month for inflammatory conditions were randomly assigned 1:1 using minimization to suspend or continue methotrexate treatment for two-weeks immediately after their COVID-19 booster. Data were analyzed using an intention to treat approach.
Results: 383 participants (mean age 59.0 years, 61% female) were randomized to either suspend or continue methotrexate arms. 61.4% (n=235) were female, 54.3% (n=208) had RA, 31.9% (n=122) psoriasis with/without arthritis. The median methotrexate dose was 20 mg/week. 94.5% (n=362) received a mRNA vaccine booster, mean 178 days after the second dose of the primary vaccination. Adherence to the intervention was high with 96.3% (n=184) and 97.4% (n=187) self-reported adherence with allocation in the suspend and continue methotrexate groups respectively.
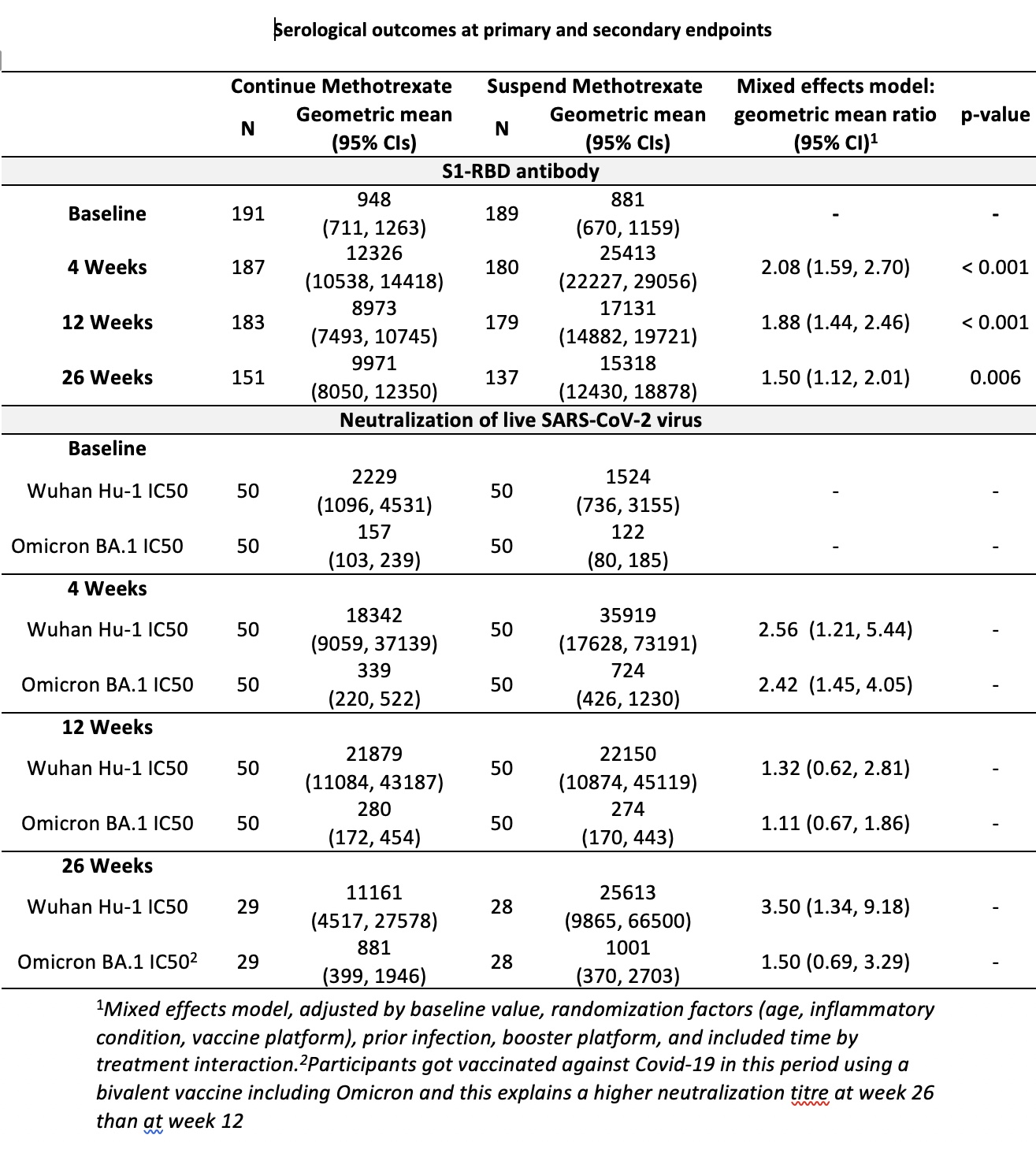
At four-weeks, the geometric mean (95% confidence interval (CI)) S1-RBD antibody level was 25,413(22,227-29,056) and 12,326(10,538-14418) U/mL in suspend and continue treatment groups respectively, with geometric mean ratio (GMR)(95%CI) 2.08(1.59-2.70), p< 0.0001, mixed-effects model. The increase in antibody response was consistent across age-groups, methotrexate doses, route, IMIDs, primary vaccination platform, and prior SARS-CoV-2 infection. Enhanced antibody responses were sustained at 12 and 26 weeks with GMR(95%CI) 1.88(1.44-2.46) and 1.50(1.12-2.01) respectively. Planned exploratory subgroup analyses suggested a greater treatment effect at higher methotrexate dose (Interaction GMR effect (95% CI) 0.67(0.47, 0.96) at 4-weeks and 0.64(0.420.96) at 12-weeks).
The Wuhan Hu-1 IC50 neutralizing antibody titer was higher in the methotrexate suspend group compared to the continue treatment group at four and 26-weeks. In a mixed-effect model, the GMR (95% CI) for Wuhan Hu-1 IC50 neutralizing antibody titer on suspending methotrexate for two-weeks was 2.56 (1.21-5.44) at 4 weeks, and 3.50 (1.34-9.18) at 26-weeks. The Omicron BA.1 IC50 cross neutralizing antibody titer was higher in the methotrexate suspend group compared to the continue treatment group at 4-weeks with GMR (95% CI) 2.42 (1.45-4.05).
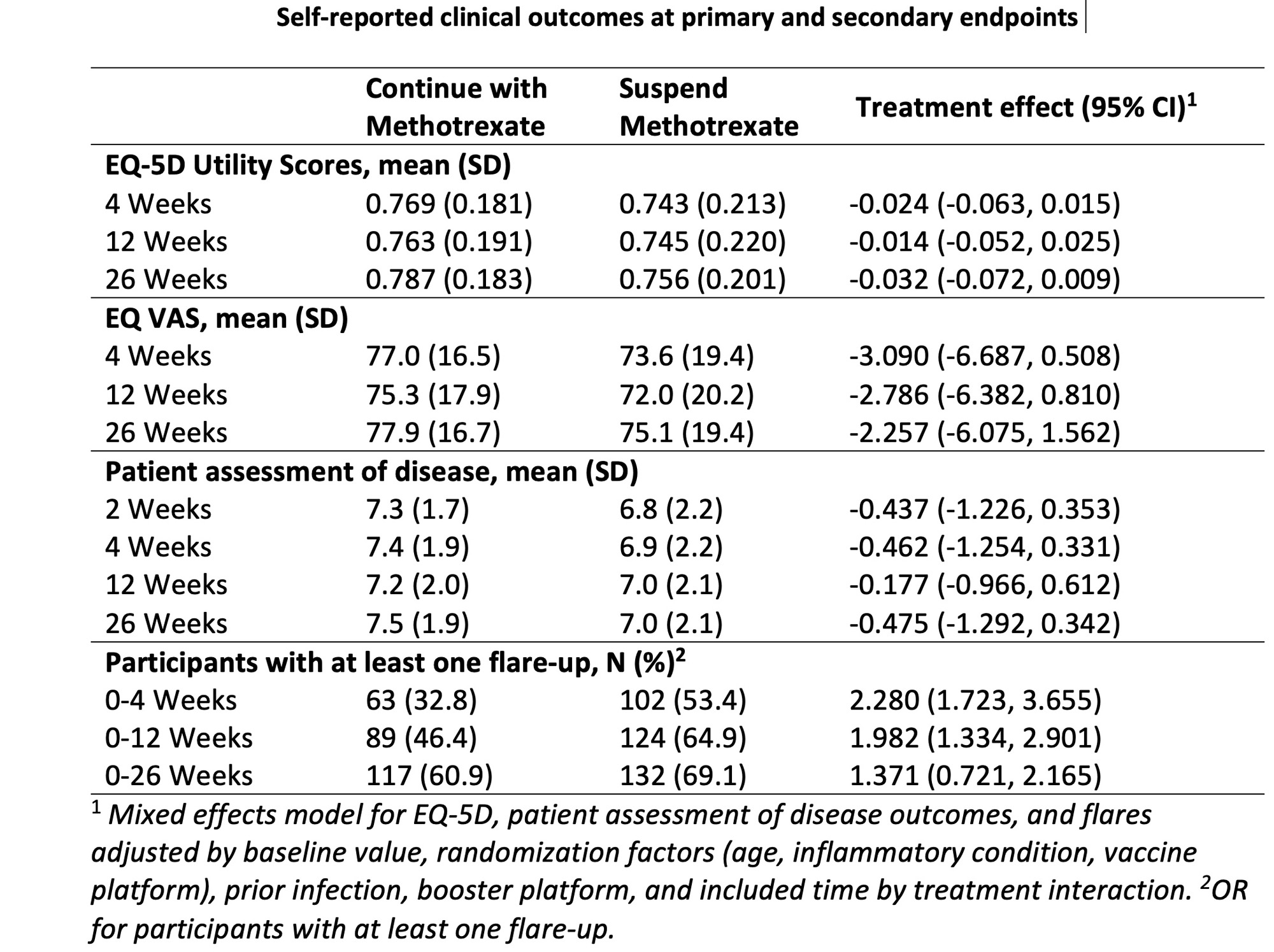
There were no differences in quality of life. Self-reported disease activity deteriorated slightly at 4-weeks in the suspend methotrexate group, but normalized by week-12.
Conclusion: Two-week interruption of methotrexate treatment for IMIDs enhanced boosting of antibody responses after COVID-19 vaccination that were sustained at 12 and 26 weeks. (Trial registration: ISRCTN11442263)
To cite this abstract in AMA style:
Abhishek A, Peckham N, Pade C, Gibbons J, Cureton L, Francis A, Barber V, Williams J, Appelbe d, Eldridge l, Julier p, Altmann d, Bluett J, Brooks T, Coates L, Rombach i, Semper A, Otter A, Valdes A, Nguyen-Van-Tam J, Williams H, McKnight A, Boyton R, Cook j. Two-week Break in Methotrexate Treatment and COVID-19 Vaccine Response. Results of the Vaccine Response on off Methotrexate (VROOM) Study, an Open Label, Prospective, Two-arm Parallel-group, Multi-center, Superiority, Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/two-week-break-in-methotrexate-treatment-and-covid-19-vaccine-response-results-of-the-vaccine-response-on-off-methotrexate-vroom-study-an-open-label-prospective-two-arm-parallel-group-multi-cen/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-week-break-in-methotrexate-treatment-and-covid-19-vaccine-response-results-of-the-vaccine-response-on-off-methotrexate-vroom-study-an-open-label-prospective-two-arm-parallel-group-multi-cen/