Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: RA causes significant morbidity and mortality. Over the last two decades, several new medications and strategies for treating RA earlier and more aggressively have become available. The objective of this study was to describe changes in RA treatment and the impact on disease outcomes over the last two decades by comparing medication use and patient-reported outcomes in a large, US-wide registry.
Methods: Patients with physician-diagnosed RA in FORWARD, The National Databank for Rheumatic Diseases, were included. Patients observed from 2008–2018 were matched 1:1 at study entry by age, sex and HAQ score to non-overlapping patients observed from 1998–2007. A random observation from each decade group was selected for demographics, disease characteristics, comorbidities, health outcomes and medications using t-test and chi-squared tests as appropriate to describe differences.
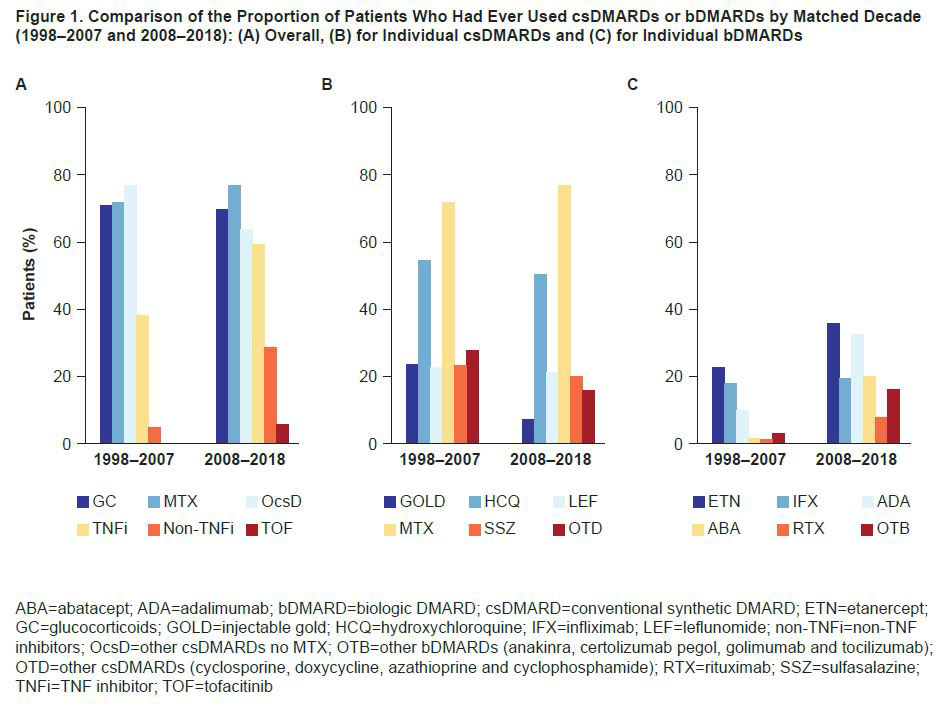
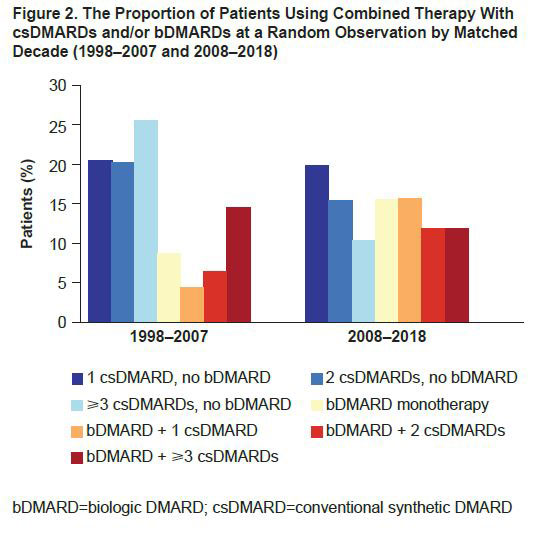
Results: We matched 8772 patients in the decade 2008–2018 with 8772 patients from the prior decade. At study entry, the time of the matching (mean [SD] calendar years 2000 [2.8] and 2012 [2.6] for respective decades), average age was 58 years, 83% were female, and mean (SD) HAQ score was 1.1 (0.7). Characterizing these decades with a random observation (Table 1), we observed slightly worse disease activity measures including pain visual analog scale, patient global score, Short Form-36 mental component summary score, quality of life and more comorbidities in the later decade, even when matching for HAQ. For individual HAQ items, better scores were observed in 2008–2018 vs 1998–2007 for items describing the performance of daily activities. There was a higher use of aids (such as canes and wheelchairs) reported in the later decade, resulting in worsened HAQ scores. MTX was the most commonly used conventional synthetic (cs)DMARD over both decades; use of other non-MTX csDMARDs was lower and use of biologic (b)DMARDs was higher in 2008–2018 vs 1998–2007 (Figure 1). Additionally, the use of combined (≥2) csDMARDs was less frequent in 2008–2018 vs 1998–2007, whereas the use of bDMARDs, either as monotherapy or in combination with ≥1 csDMARD, was higher in 2008–2018 vs 1998–2007 (Figure 2).
Conclusion: Over the last 20 years, there was a differential pattern of DMARD use between the two time periods (1998–2007 and 2008–2018), with a shift from use of non-MTX csDMARDs toward greater bDMARD use. Although matching did not allow for differences in functional disability between the two decades, patients in the latter decade had lower scores for performing daily activities and required less assistance from others but had greater use of aids such as a cane or a wheelchair. These results may contribute to a better understanding and management of disease for patients with RA.
Writing support: Catriona McKay, Caudex; funding: Bristol-Myers Squibb.
*at the time of the analysis
To cite this abstract in AMA style:
Pedro S, Dominique A, Schumacher R, Shaw Y, Wipfler K, Simon T, Michaud K. Two Decades of Changes in RA Treatment and Disease Outcomes from the United States [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/two-decades-of-changes-in-ra-treatment-and-disease-outcomes-from-the-united-states/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-decades-of-changes-in-ra-treatment-and-disease-outcomes-from-the-united-states/