Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) have significantly improved the prognosis of patients with psoriatic arthritis (PsA); however, approximately 40% of patients do not achieve complete treatment response. There is an unmet clinical need to predict which psoriatic arthritis patients have an increased likelihood of responding to TNFi therapies. Recently published studies have demonstrated that the presence of a TNFR2 gene polymorphism (rs1061622) is linked to TNFi response in patients with immune-mediated diseases, including psoriasis. Presence of the TNFR2 gene with T/T genotype shows a significantly higher rate of response to TNFi compared to G/G genotype in these patients. However, the relationship between this specific TNFR2 gene polymorphism and TNFi response in PsA patients is not known. The aim of this study was to test the association of 3 genotypes (T/T genotype as compared to the T/G and G/G genotypes) with anti TNF response in a cohort of psoriatic arthritis patients.
Methods: Patients treated with TNFi were identified from the PsA longitudinal cohort. Genomic DNA isolated from the buffy coats of these patients were used to perform restriction fragment length polymorphism (RFLP) analysis to identify T/T, T/G or G/G genotypes. TNFi response was then evaluated through chart review by two board certified rheumatologists. Non-response was defined as change of TNFi treatment due to lack of achieving low disease activity in skin or joint symptoms. Response rate was studied retrospectively and groups were matched for age, gender and BMI.
Continuous variables were summarized using means and standard deviations. Categorical variables were summarized using counts and percentages. Univariable logistic regression with each genotypes as a single predictor was built to investigate the relationship between genotypes and non-response to TNFi. Results were represented using odds ratios and 95% confidence intervals. Data management and analysis were done in R software (Version 3.5; Vienna, Austria). All tests were two-sided, with an alpha level of 0.05.
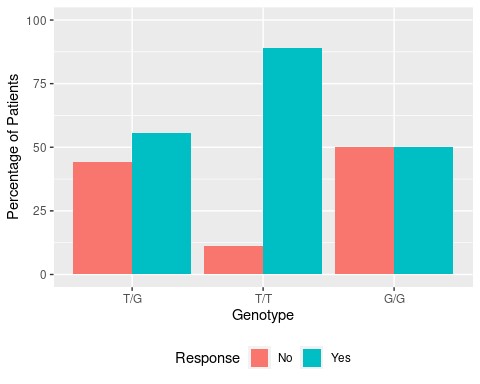
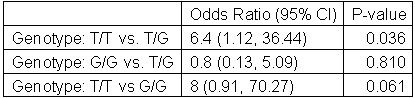
Results: 42 patients were included (50.0% female, mean age 53.6 years, mean BMI 29.7) in the analyses. Our results demonstrated that 89% of patients with T/T genotype (n=18) were responders to TNFi, as compared to only 56% of patients with T/G genotype (n=18) and 50% of patients with G/G genotype (n=6) (Figure 1). The main finding in our study was that the odds of having a response in the T/T genotype is 6.4 times the odds of having a response in the T/G genotype (OR = 6.4 (1.12, 36.44), p = 0.036) (Table 1).
Conclusion: Our results suggest that PsA patients homozygous for T genotype (T/T), as compared to the T/G or G/G genotypes, show a stronger likelihood of response to TNFi. Identifying the association between these TNFR2 gene polymorphisms and TNFi response can potentially be a valuable guide in a more personalized approach to treatment options for PsA patients. Our study suggests the potential role of pharmacogenomics profiling in predicting treatment response.
To cite this abstract in AMA style:
Rasheed S, Dunlap M, Harvey J, Brennan C, Jin Y, Chandrasekharan U, Husni M. Tumor Necrosis Factor-α Receptor 2 Polymorphisms and Response to TNF Inhibitor Therapy in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/tumor-necrosis-factor-%ce%b1-receptor-2-polymorphisms-and-response-to-tnf-inhibitor-therapy-in-patients-with-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tumor-necrosis-factor-%ce%b1-receptor-2-polymorphisms-and-response-to-tnf-inhibitor-therapy-in-patients-with-psoriatic-arthritis/