Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose : Data suggest that hydroxychloroquine (HCQ) use during systemic lupus erythematosus (SLE) pregnancies improves outcomes. In the past decade, single-center studies report that a high percentage of patients receive HCQ during pregnancy, yet HCQ use at a population level has not been studied. Therefore, we sought to describe time-trends in the use of HCQ in a large population-based cohort of pregnant women with SLE.
Methods : A cohort of pregnant women with SLE enrolled continuously in public (Medicaid, 2001-2010) or private (United Healthcare, 2004-2012) health insurance between three months prior to conception and one month after delivery was identified. Included patients were stratified based on their use of HCQ in the 3-month period immediately prior to pregnancy. Among women with SLE not using HCQ prior to pregnancy, we assessed the proportion initiating HCQ during pregnancy each calendar year. For women using HCQ prior to pregnancy, we calculated the proportion of women continuing HCQ during pregnancy each calendar year. Further, we described time-trends in the cumulative day-supply of HCQ prescriptions dispensed during pregnancy as a measure of the duration of HCQ use. Linear trend tests were conducted for proportions and total day-supply over the study years for both groups.
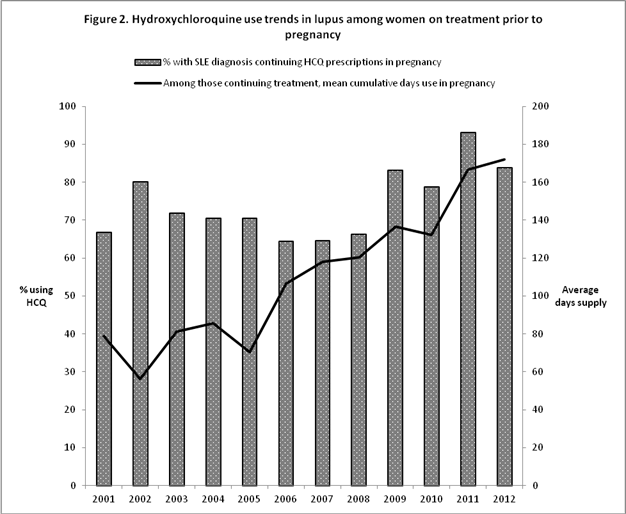
Results : A total of 4,904 women with diagnosis of SLE who became pregnant were included. Of those, 739 (15.1%) were on HCQ treatment in the 3-month period immediately prior to their pregnancy. The proportion of women initiating HCQ during pregnancy increased from 2.7% in 2001 to 11.3% in 2012 (p<0.0001, Figure 1) and the proportion of women continuing HCQ increased from 63.7% in 2001 to 83.8% in 2012 (p=0.06, Figure 2). Among women initiating HCQ, the average cumulative day-supply of HCQ prescriptions during pregnancy increased from 37 days in 2001 to 117 days in 2012 (p<0.01). Similarly, among women continuing HCQ treatment, the average cumulative day-supply of HCQ prescriptions during pregnancy increased from 79 days in 2001 to 172 days in 2012 (p<0.0001).
Conclusion: The proportion of women with SLE initiating or continuing HCQ during pregnancy increased from 2001 to 2012. Similarly, average cumulative day-supply of HCQ prescription increased during the same time frame. While these findings are encouraging, overall HCQ use during pregnancy remains low and is a potential area for clinical intervention. 
To cite this abstract in AMA style:
Bermas BL, Kim S, Huybrechts K, Hernandez-diaz S, Bateman BT, Desai RJ. Trends in Use of Hydroxychloroquine during Pregnancy in SLE Patients from 2001 to 2012 [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/trends-in-use-of-hydroxychloroquine-during-pregnancy-in-sle-patients-from-2001-to-2012/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trends-in-use-of-hydroxychloroquine-during-pregnancy-in-sle-patients-from-2001-to-2012/
