Session Information
Date: Sunday, November 8, 2020
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Infliximab and adalimumab therapy has significantly improved the prognosis of patients with non-infectious refractory uveitis. However, there is not enough evidence for the use of other anti-TNFs such as Certolizumab Pegol (CZP). Our objective was to evaluate the efficacy and safety of CZP in uveitis due to Immune-Mediated Inflammatory Diseases (IMID).
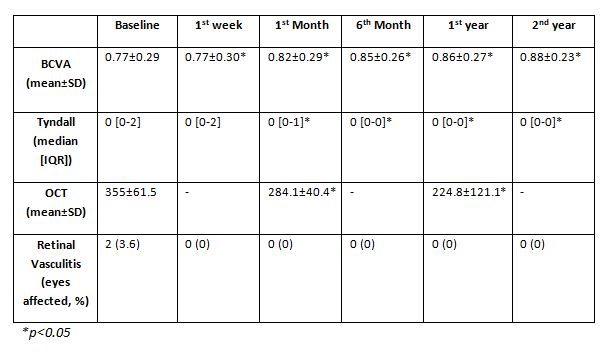
Methods: Multicenter study of 39 patients with uveitis due to IMID refractory to glucocorticoids and conventional immunosuppressants. The efficacy of CZP was evaluated with the following ocular parameters: best corrected visual acuity (BCVA), anterior chamber cells, macular thickness and presence of retinal vasculitis. Efficacy of CZP was compared between baseline, 1st week, 1st and 6th month, and 1st and 2nd year. Statistical analysis was performed with STATISTICA (Statsoft Inc. Tulsa, Oklahoma, USA).
Results: 39 patients/56 affected eyes (18 men/21 women) with a mean age of 40.5±11.9 years were studied. IMIDs included were: spondyloarthritis (n=17), psoriatic arthritis (6), Crohn (3), juvenile idiopathic arthritis (JIA) (2), Behçet (2), reactive arthritis (2), rheumatoid arthritis (1), relapsing polychondritis (1), pars planitis (1), Birdshot (1) and idiopathic uveitis (3). Uveitis pattern was as follows: anterior (n=30), posterior (4), panuveitis (3) and intermediate (2).
Previous CZP, patients received: oral prednisone (n=18) methylprednisolone bolus (1), methotrexate (22), azathioprine (10), cyclosporine (4), leflunomide (2), mycophenolate mofetil (2) and cyclophosphamide (1). 77% of patients had received previous biological therapy, with a mean of 1.6±1.2 biological drugs per patient. Gestational desire was the reason for prescribing CZP in 8 patients. CZP was administered in monotherapy in 16 patients and in the remaining 23 patients combined with conventional immunosuppressants.
After a median follow-up of 24 [6-36] months, most of the ocular variables analysed showed a rapid and significantly sustained improvement (Table). CZP was discontinued in 11 patients for the following reasons: remission (n=1), insufficient response of ocular symptoms (n=1) and limited response of extraocular manifestations (n=9). No serious adverse effects were reported.
Conclusion: CZP seems to be effective and safe in patients with refractory uveitis due to IMID.
To cite this abstract in AMA style:
Martín-Varillas J, Calvo-Río V, Sanchez-Bilbao L, Gonzalez-Mazon I, Torre I, García Martos A, Sánchez Andrade A, García Aparicio A, De dios J, Urriticoechea A, Maiz Alonso O, Veroz R, García Valle A, Rodriguez Montero S, Miguelez R, Jovani V, Hernandez Garfella M, Conesa A, Martinez Gonzalez O, Rubio Muñoz P, Peña Sainz-Pardo E, González-Gay M, Blanco R. Treatment with Certolizumab Pegol in Refractory Uveitis Secondary to Inmune-Mediated Inflammatory Diseases. Multicenter Study of 39 Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/treatment-with-certolizumab-pegol-in-refractory-uveitis-secondary-to-inmune-mediated-inflammatory-diseases-multicenter-study-of-39-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-with-certolizumab-pegol-in-refractory-uveitis-secondary-to-inmune-mediated-inflammatory-diseases-multicenter-study-of-39-patients/