Session Information
Date: Tuesday, November 12, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster III: Comorbidities
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Pulmonary complication is one of the leading causes of mortality in patients with rheumatoid arthritis (RA). Pre-existing lung disease is known as a risk factor for development of severe pulmonary events during treatment with synthetic and biologic disease-modifying anti-rheumatic drugs (DMARDs). However, it remains unknown whether risk of severe pulmonary events is higher in patients treated with biologic DMARDs (bDMARDs) than those without, in the setting of pre-existing lung disease. This study is aimed to clarify this clinical question by using single-center cohort data.
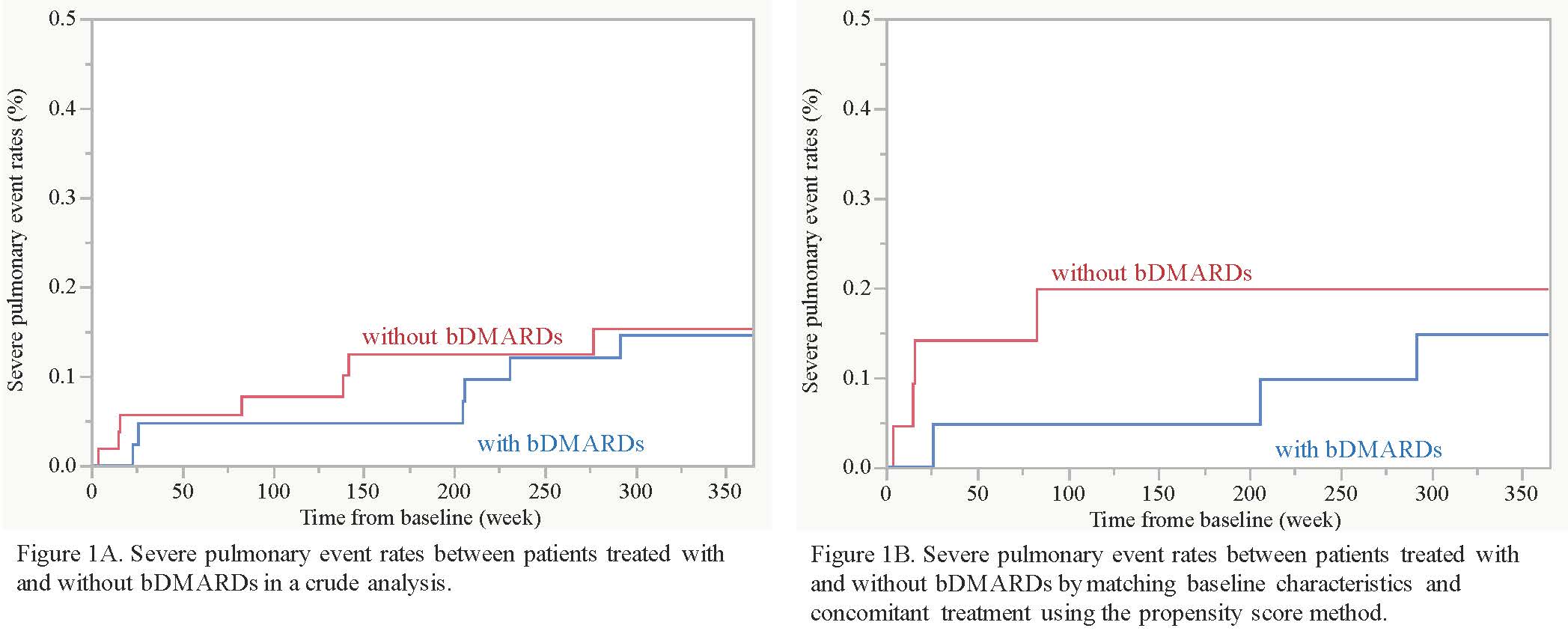
Methods: This study used RA patients registered in a cohort enrolling consecutive patients with RA at Nippon Medical School Hospital, based on the following criteria: satisfaction of 2010 ACR/EULAR classification criteria; pre-existing lung disease, including interstitial lung disease (ILD), chronic obstructive pulmonary disease (COPD), bronchial asthma, airway disease such as bronchiectasisand bronchiolitis, and lung infection such as pulmonary non-tuberculosis mycobacteria (NTM)confirmed by chest CT; and follow-up of at least 52 weeks after chest CT imaging. Severe pulmonary event was defined by newly onset or reactivation pulmonary infection, newly onset or exacerbation of ILD, and drug-induced lung injury that require therapeutic intervention. Baseline characteristics and concomitant treatmentwere compared between patients treated with and without bDMARDs, Then, baseline characteristics and concomitant treatment were matched using the propensity score method. Cumulative rates for severe pulmonary events were compared using Kalan-Meier method, and statistical difference between 2 groups was tested by log-rank test.
Results: This study analyzed 99 patients with RA and pre-existing lung disease. The median age at enrollment was 73 years, and 59% were female. Pre-existing lung diseases included airway disease in 61, ILD in 44, COPD in 22, pulmonary NTM in 4, and bronchial asthma in one.During the follow-up of 52 weeks, 45 patients received 1 or 2 bDMARDs (TNF inhibitors in 15, abatacept in 19, and tocilizumab in 13), while the remaining 54 patients did not. A total of 17 severe pulmonary events occurred in 14 patients (9 pneumonia, 5 worsening of ILD, 2 drug-induced lung injury, and one Pneumocystis jirovecipneumonia). In a crude analysis, there was no difference of cumulative severe pulmonary event rates between patients treated with and without bDMARD (15% versus 15% at 52 weeks, P = 0.81; Figure 1A). Younger age, higher KL-6, and higher anti-CCP antibody titer were significantly associated with use of bDMARDs, but methotrexate and corticosteroids were used similarly between the 2 groups. After propensity score matching by employing age, KL-6, and anti-CCP antibody titer, severe pulmonary event rates were still comparable between patients treated with and without bDMARDs (15% and 20% at 52 weeks, P = 0.54; Figure 1B).
Conclusion: Use of bDMARDs was not associated with increased risk of severe pulmonary events in RA patients with pre-existing lung disease, while this finding should be validated by independent multicenter cohorts.
To cite this abstract in AMA style:
Watanabe S, Gono T, Fukue R, Kobayashi S, Shirai Y, Takeno M, Kuwana M. Treatment with Biologic DMARDs Does Not Increase Risk of Severe Pulmonary Events in Patients with Rheumatoid Arthritis and Pre-existing Lung Disease [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/treatment-with-biologic-dmards-does-not-increase-risk-of-severe-pulmonary-events-in-patients-with-rheumatoid-arthritis-and-pre-existing-lung-disease/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-with-biologic-dmards-does-not-increase-risk-of-severe-pulmonary-events-in-patients-with-rheumatoid-arthritis-and-pre-existing-lung-disease/