Session Information
Date: Saturday, November 6, 2021
Title: Health Services Research Poster I: Lupus, Inflammatory Arthritis, & More (0128–0148)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Adalimumab (ADA) retention rates are impaired in patients (pts.) with chronic inflammatory rheumatic diseases (CIRD) due to loss of efficacy and adverse events, causing ADA discontinuation by 50% of pts. within 5 years (Neovius et al., 2015, Ann Rheum Dis). The introduction of ADA biosimilars has increased non-medical switching from originator to ADA biosimilars in rheumatologic care. This study aims at analysing treatment trajectories over two years in CIRD pts. receiving originator ADA.
Methods: CIRD pts. on originator ADA who switched to ADA biosimilar from 10/2018 onwards were identified and followed until 2020. Disease activity (ASDAS), physical function (HAQ, BASFI), and treatment changes were documented every 3 months. The four predefined treatment trajectories “continued ADA biosimilar therapy”, “back-switch to originator ADA therapy”, “switch to another biological (b) disease modifying anti-rheumatic drug (DMARD) therapy”, and “stopped bDMARD therapy /death /drop out” were used to compare pts. characteristics with different trajectories.
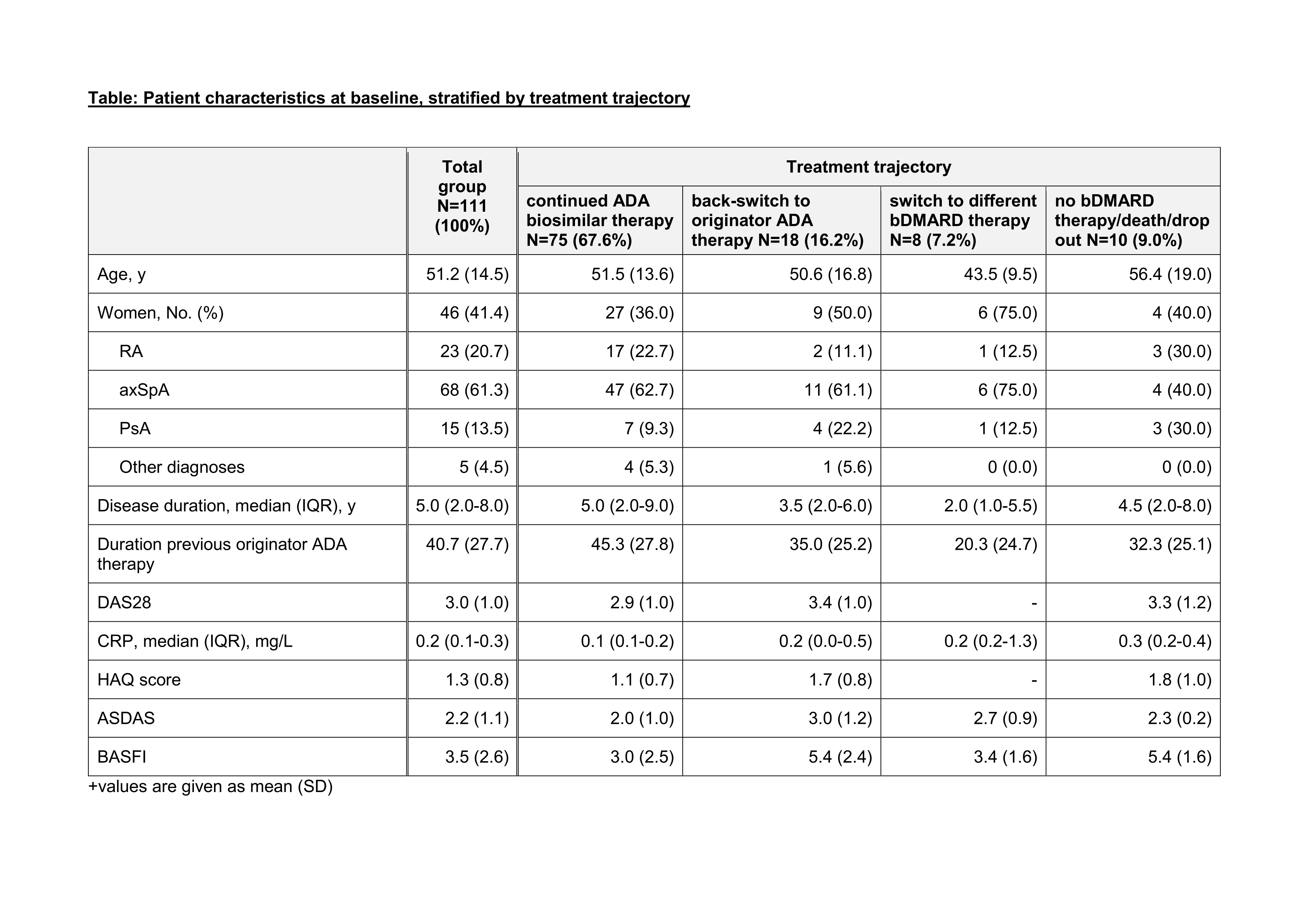
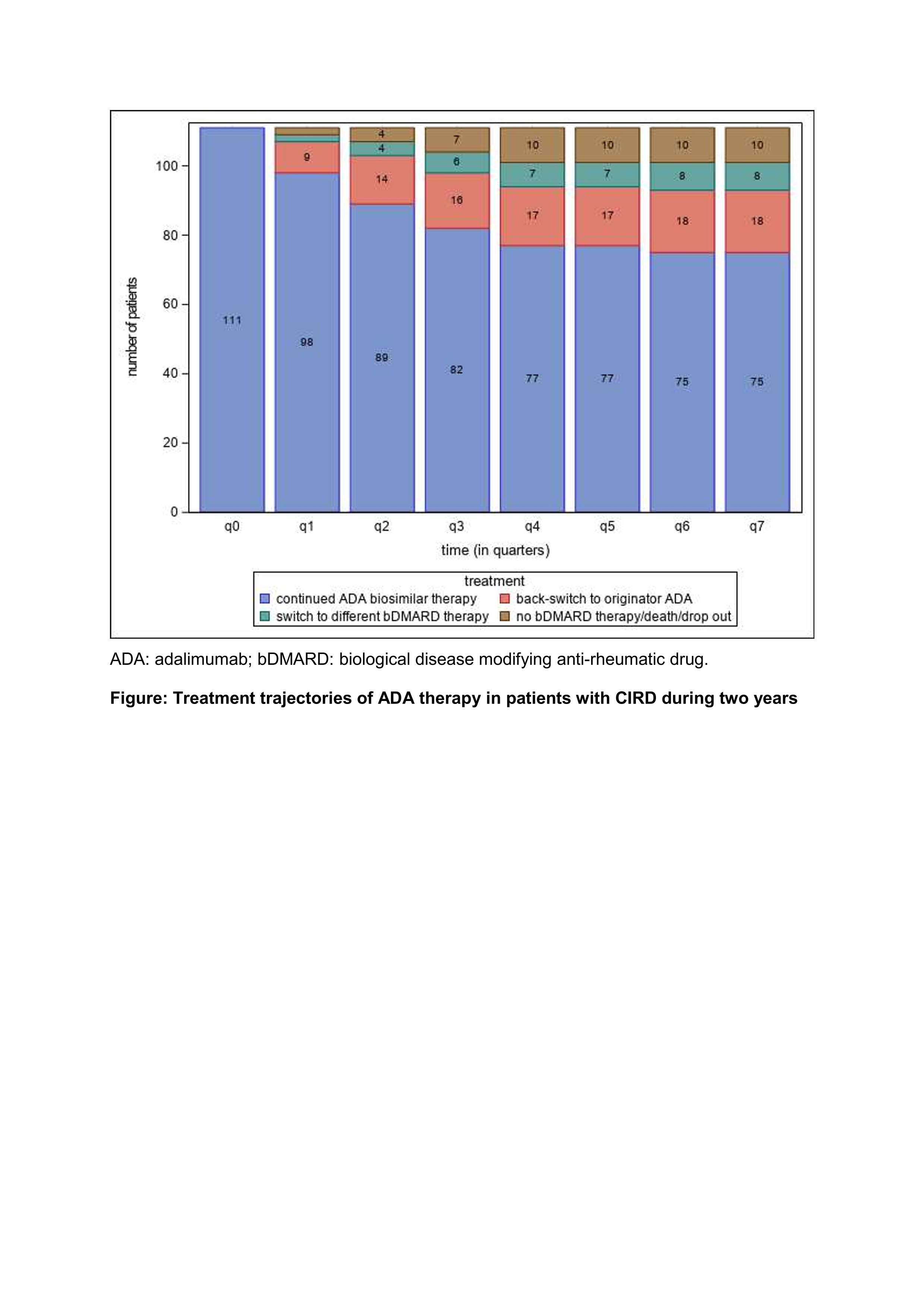
Results: 111 CIRD pts. treated with originator ADA were switched to ADA biosimilar (Table). The majority of pts. (67,6%) continued biosimilar therapy (Figure) while 16% switched back to originator ADA, 7% switched to a different bDMARD, and 9% either stopped treatment (n=9) or died (n=1). Pts. continuing ADA biosimilar were mostly male, older and/or with a longer disease duration than the back switchers to originator ADA and thoseswitching to a different bDMARD (Table). No functional impairment (HAQ, BASFI) and higher disease activity (ASDAS) were observed in switching pts. compared to pts. continuing ADA biosimilar therapy (Table). CsDMARD and glucocorticoid treatment were increased in pts. continuing ADA biosimilar therapy, while pts. switching therapy had more previous bDMARD therapies (Table).
Conclusion: Two thirds of pts. who switched to ADA biosimilar remained on this therapy for 2 years. However, as many as 16% of pts. switched back to ADA originator. Whether or to what degree nocebo effects influenced this needs further study.
Table legend on bottom: +values are given as mean (SD)
Figure: Treatment trajectories of ADA therapy in patients with CIRD during two years;
ADA: adalimumab; bDMARD: biological disease modifying anti-rheumatic drug.
To cite this abstract in AMA style:
Redeker I, Moustakis S, Tsiami S, Baraliakos X, Andreica I, Buehring B, Braun J, Kiltz U. Treatment with Adalimumab in Patients with Chronic Inflammatory Rheumatic Diseases: A Study of Treatment Trajectories on a Patient Level in Clinical Practice [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/treatment-with-adalimumab-in-patients-with-chronic-inflammatory-rheumatic-diseases-a-study-of-treatment-trajectories-on-a-patient-level-in-clinical-practice/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-with-adalimumab-in-patients-with-chronic-inflammatory-rheumatic-diseases-a-study-of-treatment-trajectories-on-a-patient-level-in-clinical-practice/