Session Information
Date: Sunday, November 10, 2019
Title: 3S089: Health Services Research I: Clinical Perspectives (951–956)
Session Type: ACR/ARP Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: In RA patients not meeting treat-to-target goals despite treatment with a biologic (b)DMARD, ACR guidelines1 recommend using other targeted immunomodulators (TIM): TNF-α inhibitor (TNFi), non-TNFi biologic (b)DMARDs, or a Janus kinase inhibitor (JAKi). Understanding treatment disposition and persistency can help optimize next treatment selection among Medicare recipients who initiated first bDMARD. We described treatment patterns and persistency in Medicare RA patients on first bDMARD.
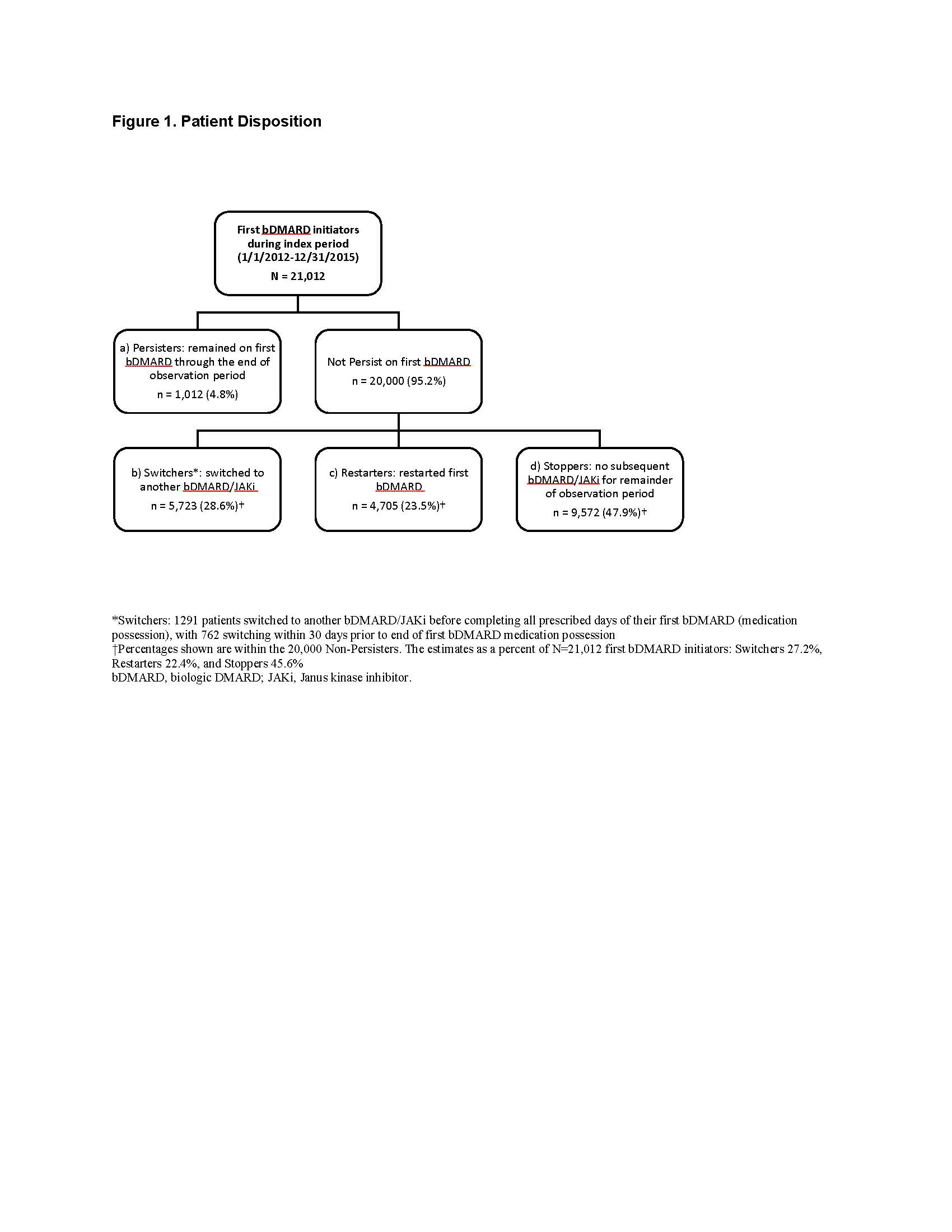
Methods: In 20% sample of Medicare fee-for-service beneficiaries, the study population comprised RA patients (≥1 RA claims + ≥1 DMARD claims) who initiated (index date) their first bDMARD between 01/2012 and 12/2015 (no bDMARD use ≥12 months pre-index), without cancer or non-RA autoimmune disease. Subsequent treatment patterns were described during a follow-up period ending 12/2016, with censoring at death or end of Medicare coverage. Patients were grouped as Persisters (persisted on their first bDMARD) or Non-Persisters (did not persist), the latter being divided into Switchers (switched to another TIM), Restarters (restarted the first bDMARD after a gap), and Stoppers (stopped TIMs altogether). Persistency loss was estimated through Kaplan-Meier time-to-event analysis, with the event being discontinuation or first gap in initial bDMARD coverage exceeding 60 days past next-refill due date. Persistency was estimated for all patients and stratified by Non-Persisters, and then further by Switchers, Restarters, Stoppers.
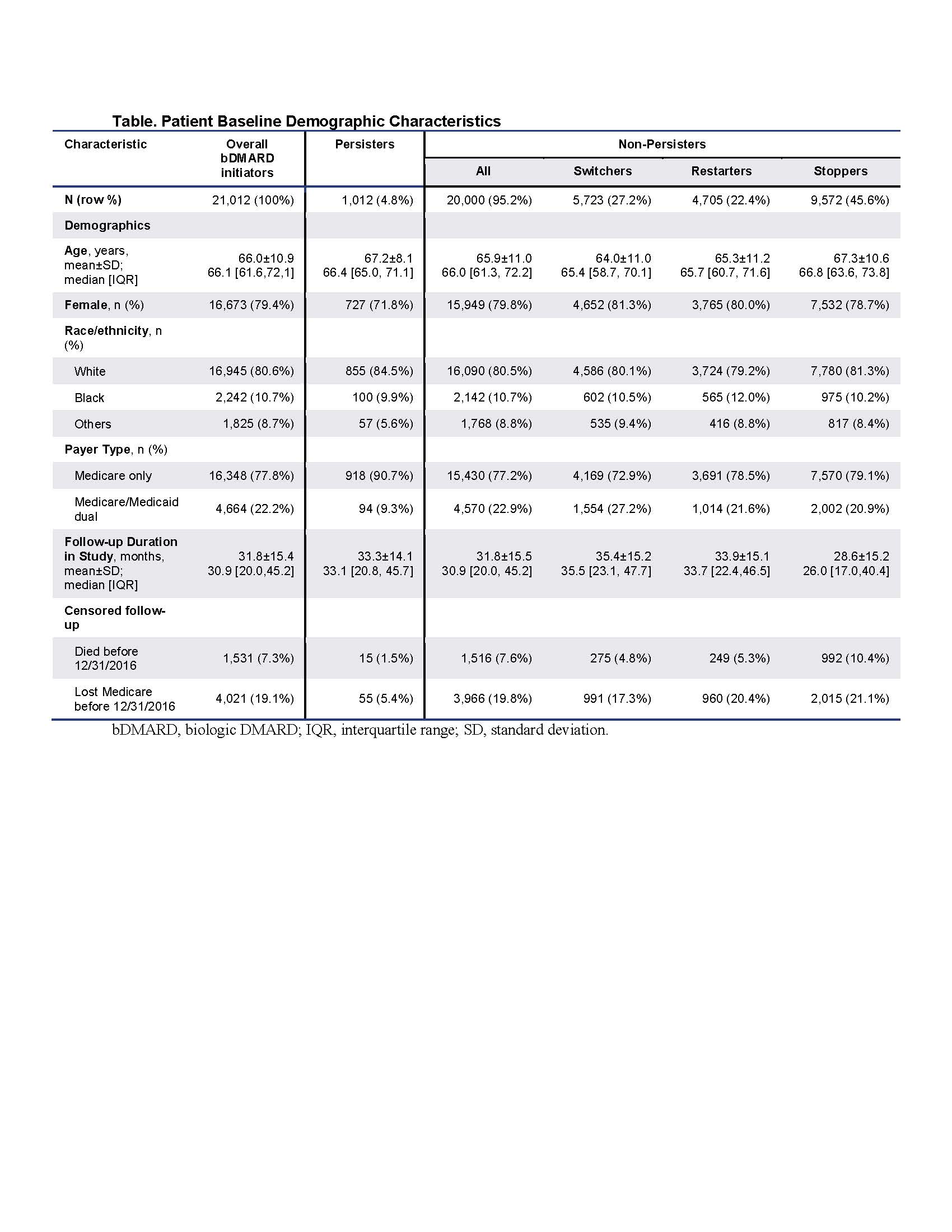
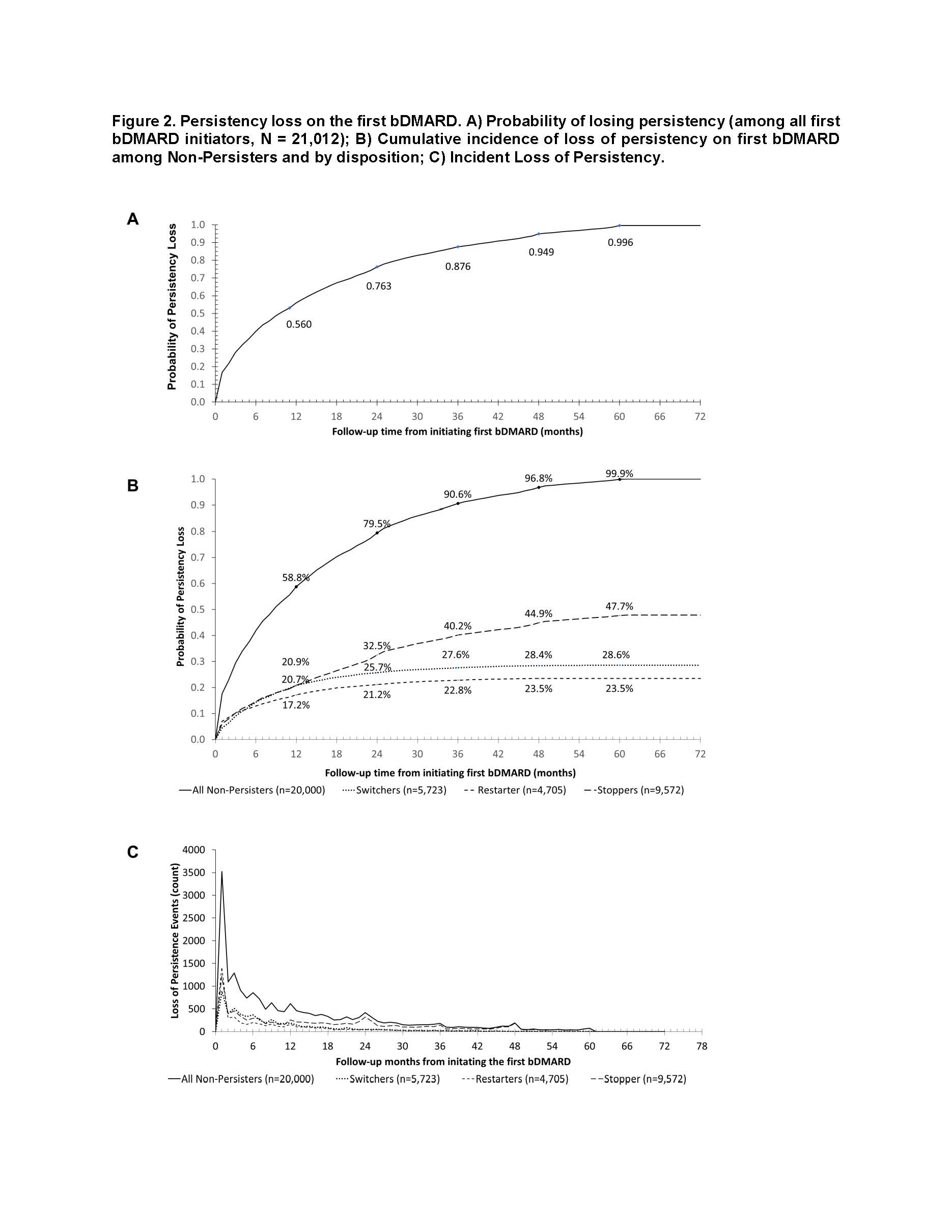
Results: The sample of 10,314,539 enrollees contained 154,311 RA patients, including 21,012 patients initiating bDMARD (52% intravenous, 48% subcutaneous): 79% female with mean age 66 years (Table). The sample consisted of 1,012 (4.8%) Persisters, 5,723 (27.2%) Switchers, 4,705 (22.4%) Restarters, and 9,572 (45.6%) Stoppers (Figure 1). Among all bDMARD initiators, 22.0% lost persistency by month 2 and 56.0% by month 12 (Figure 2a). Within Non-Persisters, the probability of losing persistency at month 12 was 58.9% overall, 72.4% for Switchers, 73.0% for Restarters, and 43.8% for Stoppers (Figure 2b). Among Non-Persisters, incident events peaked at months 1, 3, 6, 9, 12, 24, 36, 48, and 60; with contributions to persistency loss similar for all groups until month 12, after which Stoppers largely drove ongoing loss of persistency (Figure 2b, c).
Conclusion: After initiating their first bDMARD, Medicare beneficiaries with RA experienced substantial treatment interruptions, demonstrating deviations from the standard treatment-escalation approach described in the ACR guidelines. Over half the patients lost persistency on first bDMARD by year 1. Moreover, almost half of those who lost persistency on first bDMARD remained off TIM therapy altogether, suggesting a substantial unmet need for treatment in this patient population that warrants further investigation.
References
- Singh JA, et al. Arthritis Rheumatol 2016;68:1-26.
To cite this abstract in AMA style:
Dore R, Chang L, Ji Y, Li S, Antonova J, Genovese M. Treatment Patterns and Persistency Following the First Biologic DMARD in Patients with Rheumatoid Arthritis: Real-World Analysis of 2012–2016 US Medicare Data [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/treatment-patterns-and-persistency-following-the-first-biologic-dmard-in-patients-with-rheumatoid-arthritis-real-world-analysis-of-2012-2016-us-medicare-data/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-patterns-and-persistency-following-the-first-biologic-dmard-in-patients-with-rheumatoid-arthritis-real-world-analysis-of-2012-2016-us-medicare-data/