Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Adult Still’s disease (ASD), including adult-onset Still’s disease and carry-over systemic juvenile idiopathic arthritis (sJIA), is treated with glucocorticoids (GC) with or without immunosuppressive drugs; however, some patients fail to respond to conventional treatments or relapse upon GC dose tapering. Intravenous tocilizumab (TCZ-IV), an IL-6 receptor inhibitor, was approved for the treatment of ASD in Japan in May 2019 and has been used nationwide. The objective of this study was to describe the treatment patterns in patients with ASD in clinical practice and assess the changes in GC dose after TCZ-IV administration.
Methods: This retrospective cohort study used claims data from the Medical Data Vision Co., Ltd. (MDV) database. All patients aged ≥16 years with ≥1 International Classification of Diseases 10th revision (ICD-10) code M061 (ASD) or M082 (sJIA) between January 2017 and March 2021 constituted cohort 1, whereas those who initiated TCZ-IV between May 2019 and March 2021 constituted cohort 2. The index date was the initial TCZ-IV prescription date for cohort 2. In cohort 1, the time course of the treatment pattern for patients with ASD was analyzed every 3 months. In cohort 2, the proportion of patients on oral GC (OGC) of ≤5 mg/day 1 year after TCZ-IV initiation (primary endpoint), TCZ-IV retention rate, and administration interval of TCZ-IV were analyzed. The statistical results are described.
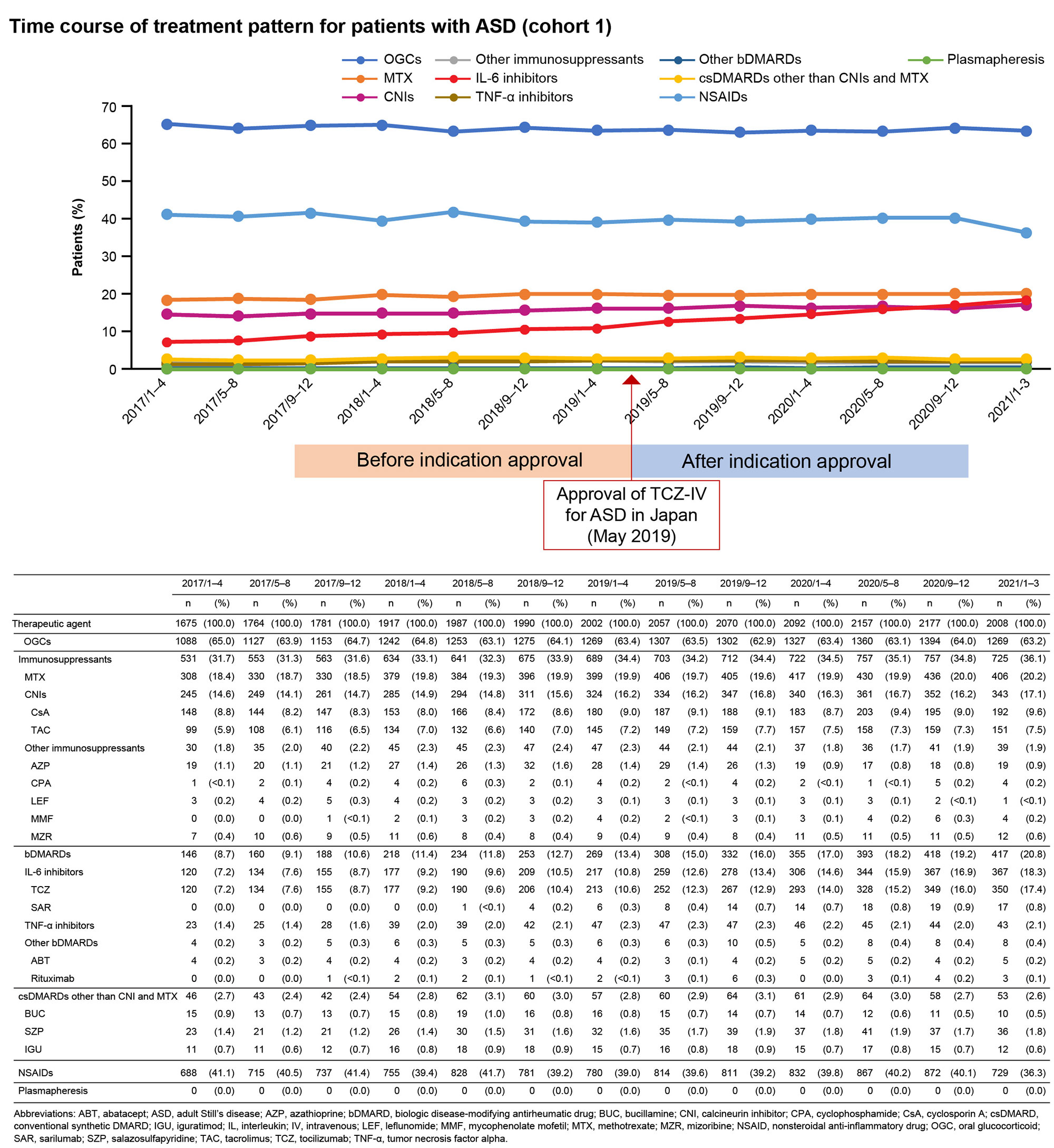
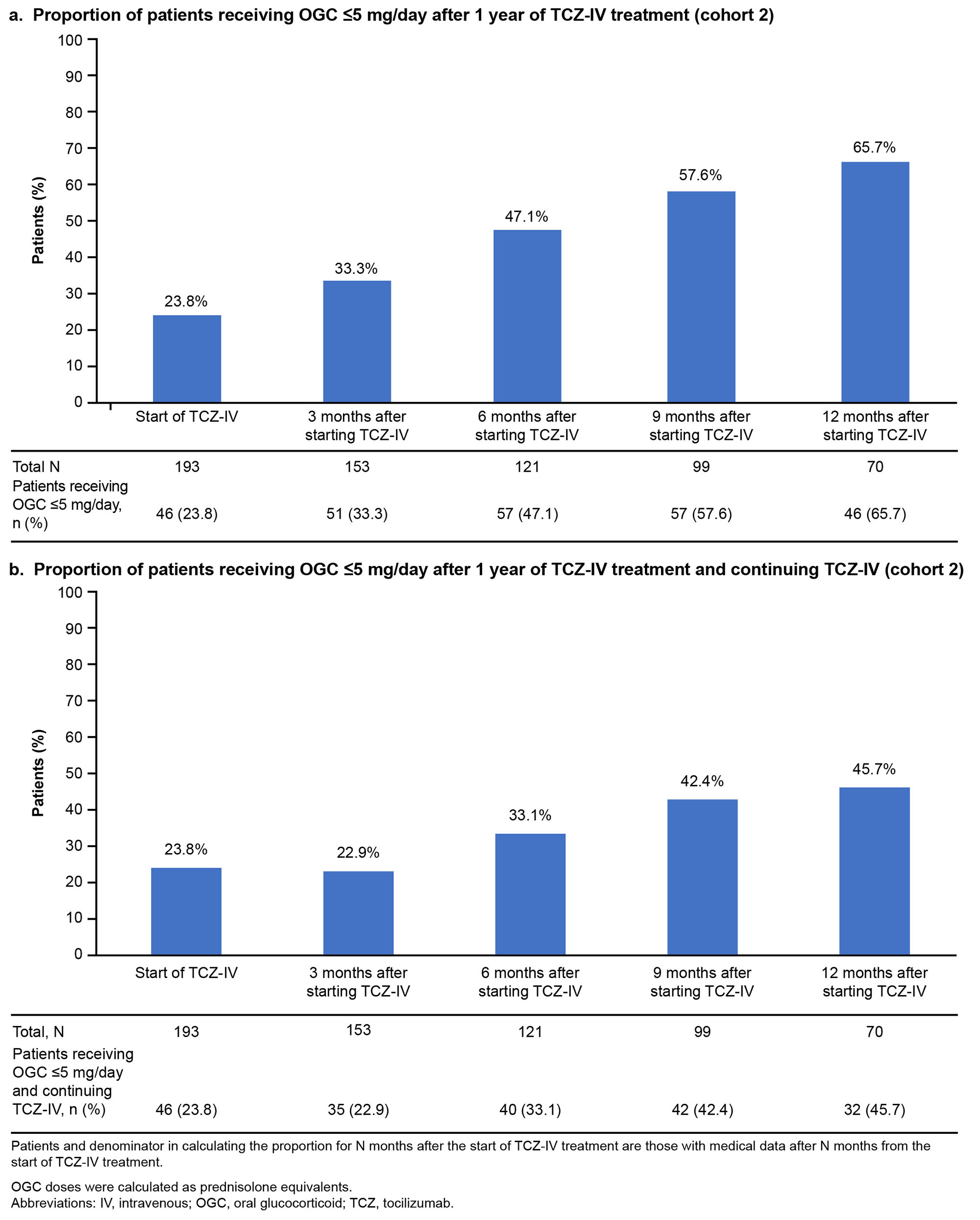
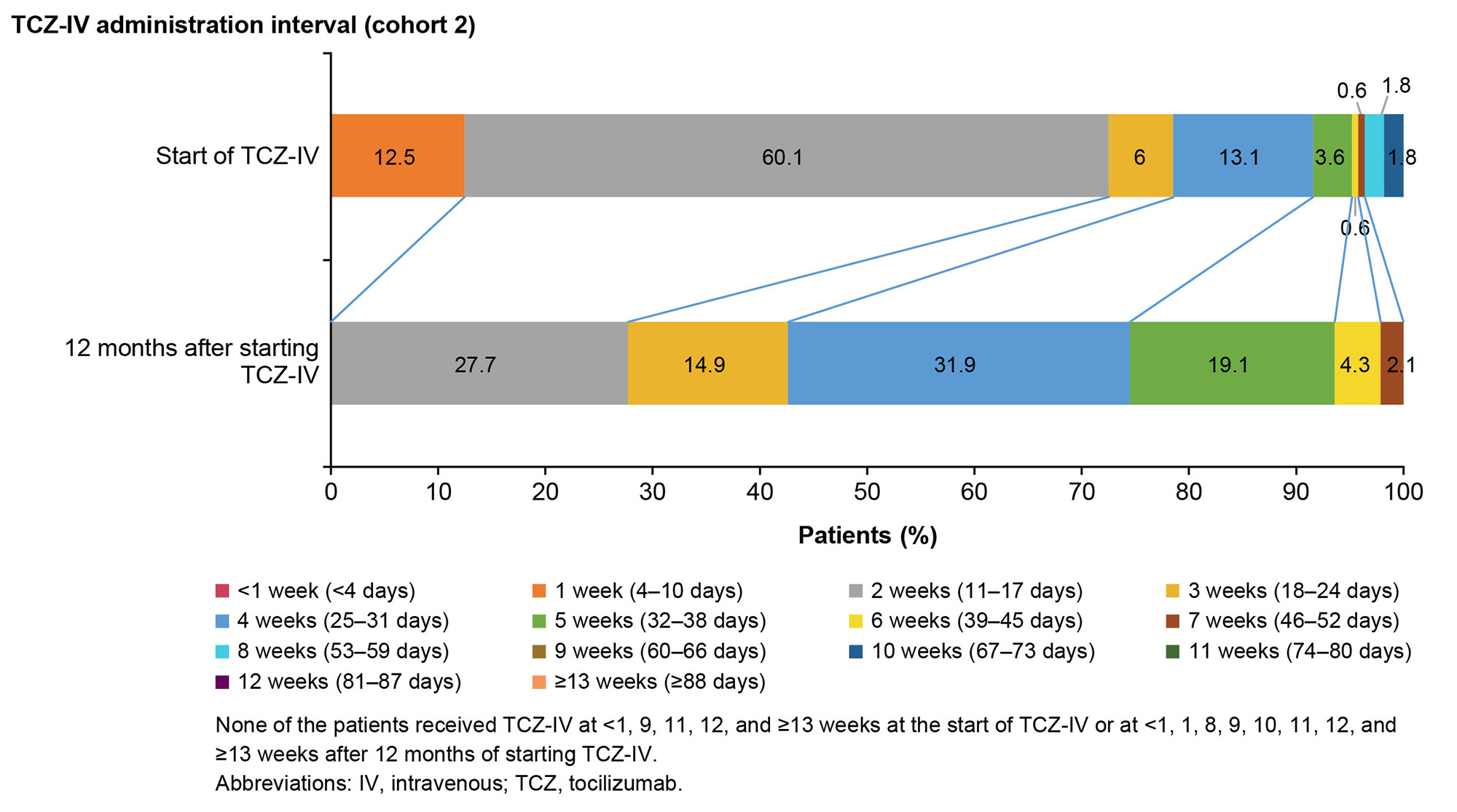
Results: Overall, 4281 and 193 patients with ASD were included in cohorts 1 and 2, respectively. Between January and March 2021, OGCs, NSAIDs, methotrexate, IL-6 inhibitors (mainly TCZ-IV), and calcineurin inhibitors were prescribed to 63.2%, 36.3%, 20.2%, 18.3%, and 17.1% of patients, respectively (cohort 1; Fig 1). The prescription rate of IL-6 inhibitors increased by 7.5% during January–March 2021 (post–TCZ-IV approval) compared with that during January–April 2019 (pre–TCZ-IV approval). The mean (standard deviation) age of patients prescribed TCZ-IV (cohort 2) was 55.1 (20.9) years, and 75.6% were female. At the index date, OGCs were prescribed to 84.5% of patients (≤5 mg/day [including those not prescribed OGCs]: 23.8%; >5 mg/day: 76.2%); 20.7% and 16.1% of patients were prescribed methotrexate and NSAIDs, respectively. One year after TCZ-IV treatment, the proportion of patients who received ≤5 mg/day of OGCs increased to 65.7%, and 45.7% of patients who continued on TCZ-IV received ≤5 mg/day of OGCs (Fig 2). The 12-month retention rate of TCZ‑IV estimated by the Kaplan-Meier method was 73.6%. At the start of treatment, 60.1% of patients received TCZ-IV at a 2-week interval. Among patients continuing treatment at 1 year, 27.7%, 14.9%, 31.9%, and 19.1% received TCZ-IV at 2-, 3-, 4-, and 5-week intervals, respectively (Fig 3).
Conclusion: The use of IL-6 receptor inhibitors increased in patients with ASD following TCZ‑IV approval for ASD (18.3% in MDV during January–March 2021). This study demonstrates the potential GC-sparing effect of TCZ-IV in patients with ASD.
b. Proportion of patients receiving OGC ≤5 mg/day after 1 year of TCZ-IV treatment and continuing TCZ-IV (cohort 2)
To cite this abstract in AMA style:
Kaneko Y, Kameda H, Ikeda K, Yamashita K, Ozaki R, Tanaka Y. Treatment Pattern and Changes in Oral Glucocorticoid Dose After Tocilizumab Treatment in Patients with Adult Still’s Disease: An Analysis of a Japanese Claims Database [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/treatment-pattern-and-changes-in-oral-glucocorticoid-dose-after-tocilizumab-treatment-in-patients-with-adult-stills-disease-an-analysis-of-a-japanese-claims-database/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-pattern-and-changes-in-oral-glucocorticoid-dose-after-tocilizumab-treatment-in-patients-with-adult-stills-disease-an-analysis-of-a-japanese-claims-database/