Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: In recent years, IL-23 inhibitors (IL23i)-have emerged as promising therapeutic options for psoriatic arthritis patients (PsA). Clinical trials have demonstrated their efficacy in both treatment-naïve patients and those with an inadequate response to TNF inhibitors 1-2 . However, real-world data on their effectiveness and long-term drug survival remain limited. This study aimed to evaluate the effectiveness, drug survival and reasons for therapy discontinuation of two IL-23i – Guselkumab and Risankizumab- in a cohort of PsA patients, reflecting real-world clinical practice.
Methods: We conducted a retrospective study of PsA patients, diagnosed according to the CASPAR criteria, who initiated treatment with Guselkumab or Risankizumab between 2019 and July 2024. Data was extracted from medical records and included demographic and clinical parameters, along with disease activity scores – DAPSA and PASI- assessed at four time points during follow up: baseline (treatment initiation), 6 months, 12 months and the last follow up visit. IL-23i effectiveness was evaluated by means of DAPSA score with DAPSA≤14 indicating good clinical response. Reasons for treatment discontinuation were documented, and drug survival was analyzed using Kaplan-Meier plots.
Results: A total of 61 PsA patients were included (Table 1). At baseline, the majority (57%) had moderate to high disease activity (DAPSA >14), while 18% were in remission (DAPSA ≤4) but had active skin involvement (mean PASI 6.75). For most patients (80%), IL-23 inhibitors were the fourth or later b/tsDMARDs used, indicating a difficult-to-treat population. By 6 months, 43% of patients achieved a DAPSA score ≤14, of whom 52% had a baseline DAPSA ≤14. This proportion declined to 30% by 12 months. Treatment discontinuation occurred in 30% of patients at 6 months and in 54% at 12 months. The primary reasons for discontinuation were persistent joint disease (65% at 6 months and 42% at 12 months) and combined joint and skin non-response (18% at 6 months, 24% at 12 months). Only 9% of patients discontinued therapy due to isolated skin disease activity.Overall, 78% of patients discontinued treatment, with a median duration of 9.4 months (range: 4.9–24.4 months).No significant differences were observed between Guselkumab and Risankizumab treatment groups.
Conclusion: In this real-world cohort of biologic-experienced, difficult-to-treat PsA patients, IL-23 inhibitors demonstrated modest effectiveness and low treatment persistence. The high discontinuation rate—primarily due to insufficient joint disease control—underscores the need for alternative therapeutic strategies in this challenging patient population.Bibliography:1. Deodhar, Atul et al. Lancet. 2020. 2. Östör, Andrew et al. Annals of the rheumatic diseases. 2022.
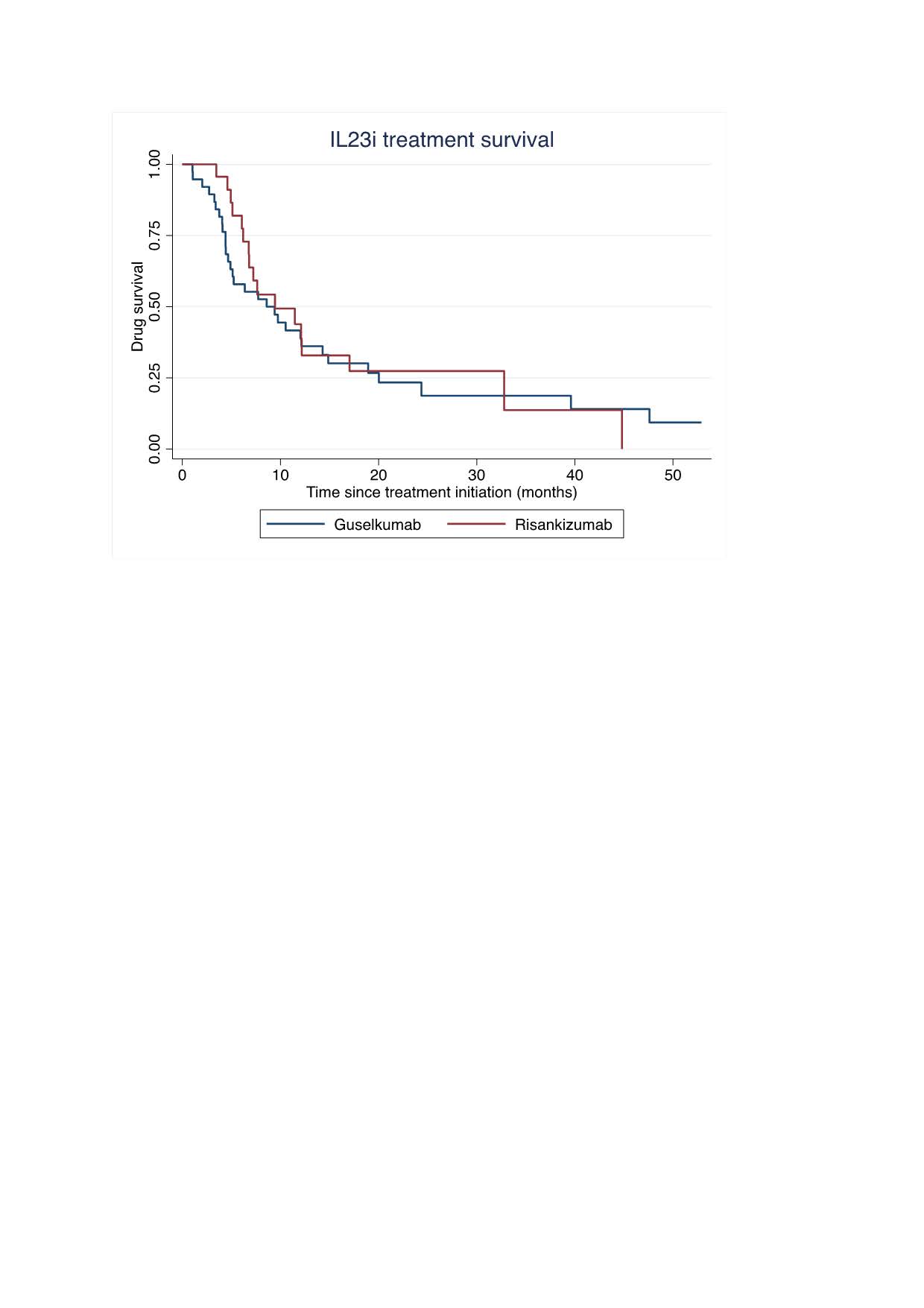 Table 1. Clinical characteristics of PsA patients at baseline
Table 1. Clinical characteristics of PsA patients at baseline
To cite this abstract in AMA style:
Rosenberg D, capelusnik D, Shtrozberg S, Elkayam O, Tzemach R. Treatment Outcome and Persistence of IL-23 Inhibitors in Real World Cohort of PsA Patients- A Retrospective Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/treatment-outcome-and-persistence-of-il-23-inhibitors-in-real-world-cohort-of-psa-patients-a-retrospective-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-outcome-and-persistence-of-il-23-inhibitors-in-real-world-cohort-of-psa-patients-a-retrospective-study/

.jpg)