Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Despite major advances in the understanding, diagnosis, and treatment of Still’s disease (including Systemic Juvenile Idiopathic Arthritis, sJIA), many patients experience a refractory course. Among potential organ-involvements, sJIA-associated lung disease (sJIA-LD) has arisen as a major comorbidity, prompting some patients to seek stem cell transplantation. Herein, we report the safety and efficacy of an investigational bi-specific IL-1b/IL-18 neutralizing antibody (aIL-1b/18, MAS825) in 7 refractory sJIA patients.
Methods: All patients received bimonthly MAS825 infusions via single-patient extended-use protocols at The Children’s Hospital of Philadelphia (CHOP), University of Pittsburgh, or Hackensack University Medical Center. Parents/guardians provided informed consent for a registry study approved by The CHOP Institutional Review Board. Clinical, laboratory, radiologic, and pathologic information were extracted from electronic medical records.
Results: Disease onset was observed between 1.25 and 11 years of age. All patients met provisional classification criteria for refractory sJIA prior to MAS825 treatment. Other baseline characteristics included a systemic course with at least one Macrophage Activation Syndrome (MAS) episode (7/7), persistent itchy rash (6/7), sJIA-LD (6/7), and peak total IL-18 levels from 77,000 to >650,000 pg/mL. All patients had received high-dose glucocorticoids, IL-1 blockade, and at least one JAK inhibitor prior to MAS825 (Table 1).Six of 7 patients remained on MAS825 at the time of data acquisition, with a duration of 0.9 to 3.6 years exposure. One patient stopped MAS825 after 4 months for lack of efficacy. Of the 6 patients remaining on MAS825, all made substantial systemic glucocorticoid dose reductions, and 5 weaned off entirely (Figure 1). All showed a substantial reduction or discontinuation of other Still’s therapy. Five of 6 patients’ Still’s biomarkers improved and had clinically significant functional improvement (physician global and/or pulmonary function test). Chest CT’s were stable or improved in 5/6 (Figure 2). No infusion reactions were reported. One patient developed pseudomonal line infection. Another was found to have S. Aureus vertebral osteomyelitis 5 months after MAS825 initiation. Symptoms and inflammatory markers initially improved with antibiotics and holding MAS825, but MAS soon flared. Re-initiation of MAS825 with antibiotics led to clinical improvement, but the infection has required long-term antibiotic treatment.
Conclusion: In this highly refractory cohort, MAS825 was overall well-tolerated, and 5/7 patients showed clinical improvement and significant reduction in glucocorticoid and other Still’s therapies. These data strongly support prospective trials, with careful monitoring particularly for invasive bacterial infection, in this immunosuppressed population with life-threatening sJIA disease. Acknowledgment: All patients were provided MAS825 via Managed Access Program. Novartis has conducted a scientific accuracy and intellectual property review of this abstract, but has not otherwise instigated or influenced the content of this publication.
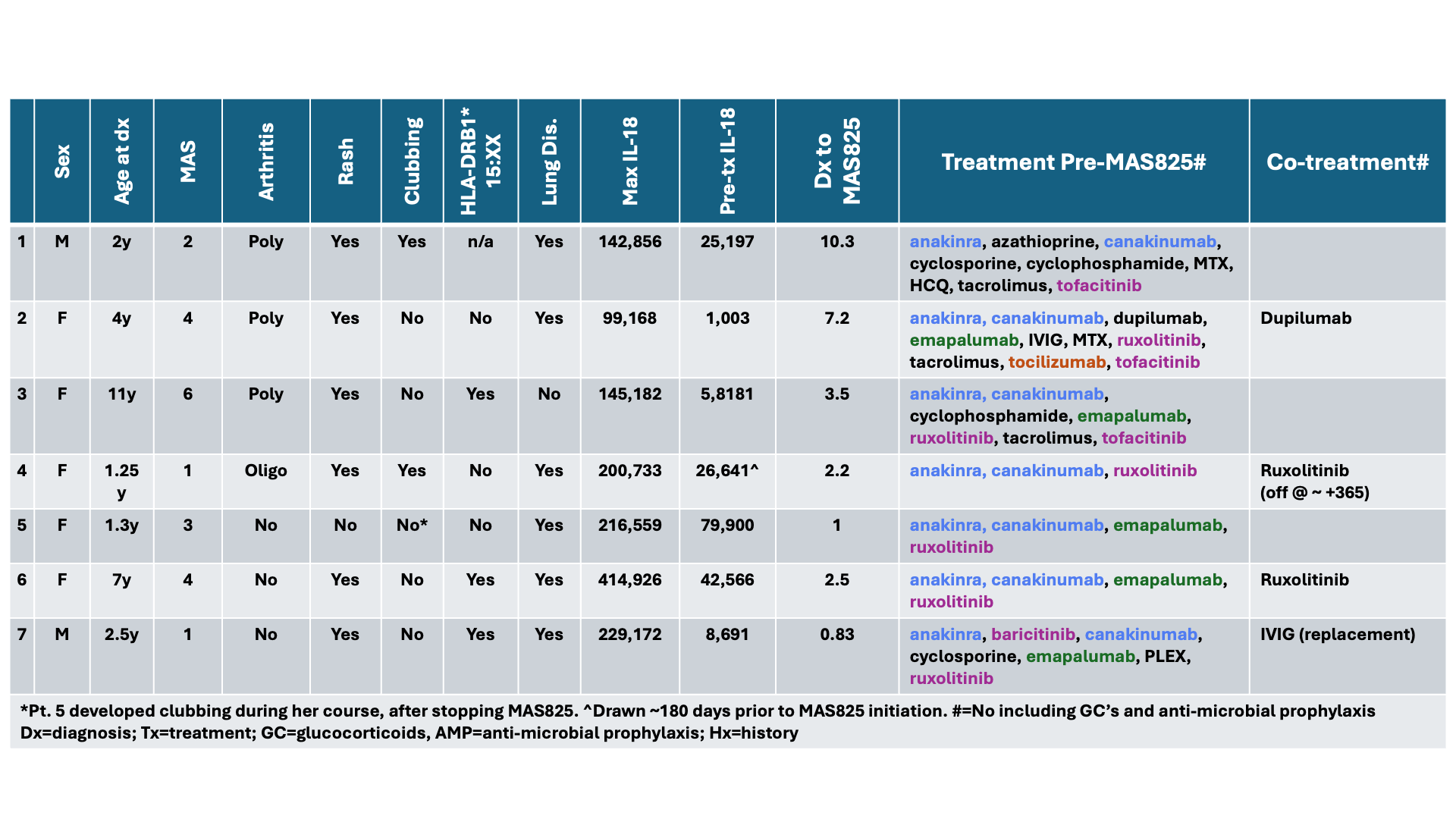 Table 1: Patient demographics and medications relative to MAS825 initiation
Table 1: Patient demographics and medications relative to MAS825 initiation
.gif) Figure 1: Steroid exposure (rolling 7-day average) relative to MAS825 initiation for patients 1-5.
Figure 1: Steroid exposure (rolling 7-day average) relative to MAS825 initiation for patients 1-5.
.gif) Figure 2: A) ferritin values relative to MAS825 initiation. B) Pre- and Post- MAS825 disease activity markers, physician global assessment, and DLCO [Hb] % Ref of patient’s pulmonary function tests. (Median, interquartile-range).
Figure 2: A) ferritin values relative to MAS825 initiation. B) Pre- and Post- MAS825 disease activity markers, physician global assessment, and DLCO [Hb] % Ref of patient’s pulmonary function tests. (Median, interquartile-range).
To cite this abstract in AMA style:
Carol H, Manglani M, Noor A, Behrens E, Burnham J, Lee J, Moreno J, Chop A, Cetin Gedik K, Poholek C, Janow G, Waqar-Cowles L, Canna S. Treatment of Refractory Still’s/Systemic Juvenile Idiopathic Arthritis Lung Disease with the bi-specific IL-1Beta/IL-18 neutralizing antibody MAS825 [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/treatment-of-refractory-stills-systemic-juvenile-idiopathic-arthritis-lung-disease-with-the-bi-specific-il-1beta-il-18-neutralizing-antibody-mas825/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-of-refractory-stills-systemic-juvenile-idiopathic-arthritis-lung-disease-with-the-bi-specific-il-1beta-il-18-neutralizing-antibody-mas825/
