Session Information
Date: Saturday, November 16, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: CD4+ cytotoxic T lymphocytes (CD4+ CTLs), plasmablasts, and selected other cells of the T and B cell lineages have been identified as cellular linchpins of IgG4-RD. Both CD4+CTLs and plasmablasts as well as plasma cells, DN3 B cells, CD8+ T cells, and NK cells, express SLAMF7, a cell surface protein involved in cell-cell interactions, cytotoxicity, and cell survival. An abundance of cells bearing SLAMF7 are seen within lesional tissues in IgG4-RD. We hypothesized that elotuzumab (Empliciti, Bristol-Myers Squib), a monoclonal antibody directed against SLAMF7, might be an effective treatment for inducing and maintaining remission in IgG4-RD. We report here the results from the first part of a two-part phase 2 trial.
Methods: The first part of the trial, designed to determine the safety and the optimal dosing regimen, originally planned to enroll 12 patients in 2 cohorts. Six patients would receive 4 weekly infusions (10 mg/kg); and six would receive 8 infusions over 10 weeks. All patients would also receive a 10-week course of prednisone with a standardized taper, beginning at 40 mg/day. The primary safety endpoint was the proportion of participants in each cohort who experienced at least one Grade 3 or higher adverse event.
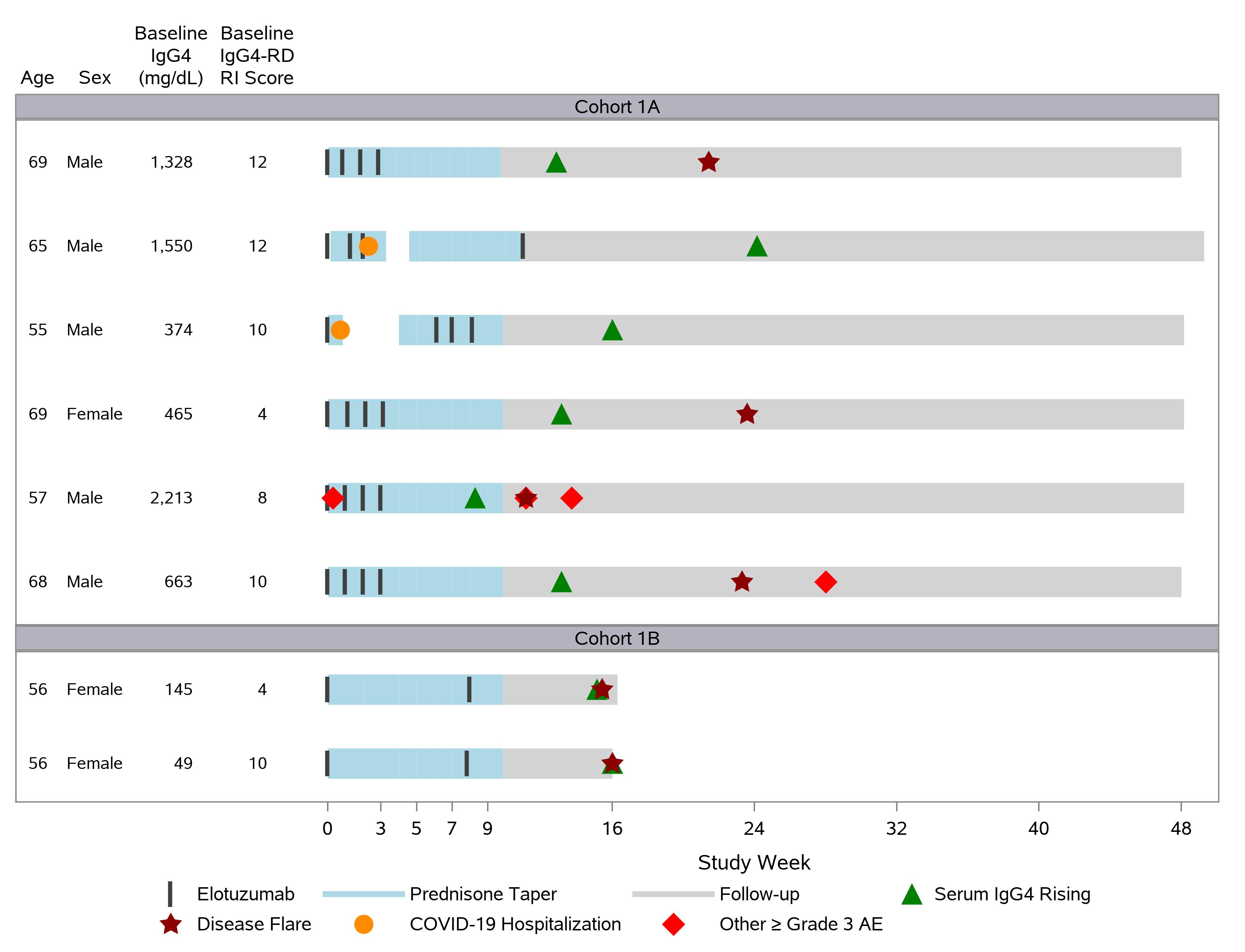
Results: Enrollment began on November 8, 2021. Over the course of the trial, a total of 8 patients were enrolled (5 male, 3 female; mean age 62 years). Their baseline disease characteristics are shown in Figure 1. Two of the first 3 patients enrolled developed Grade 3 COVID infections, leading to hospitalization. These early COVID infections, which occurred during the height of Delta variant SARS-CoV-2 infections in the U.S., led to suspension of the trial so that adequate prophylactic measures could be implemented. Added safety measures included mandatory vaccination according to current U.S. Centers for Disease Control guidelines, tixagevimab/cilgavimab (Evusheld) administration, and testing for current COVID infection prior to each elotuzumab treatment. Following resumption of the trial, enrollment in the first cohort was completed.
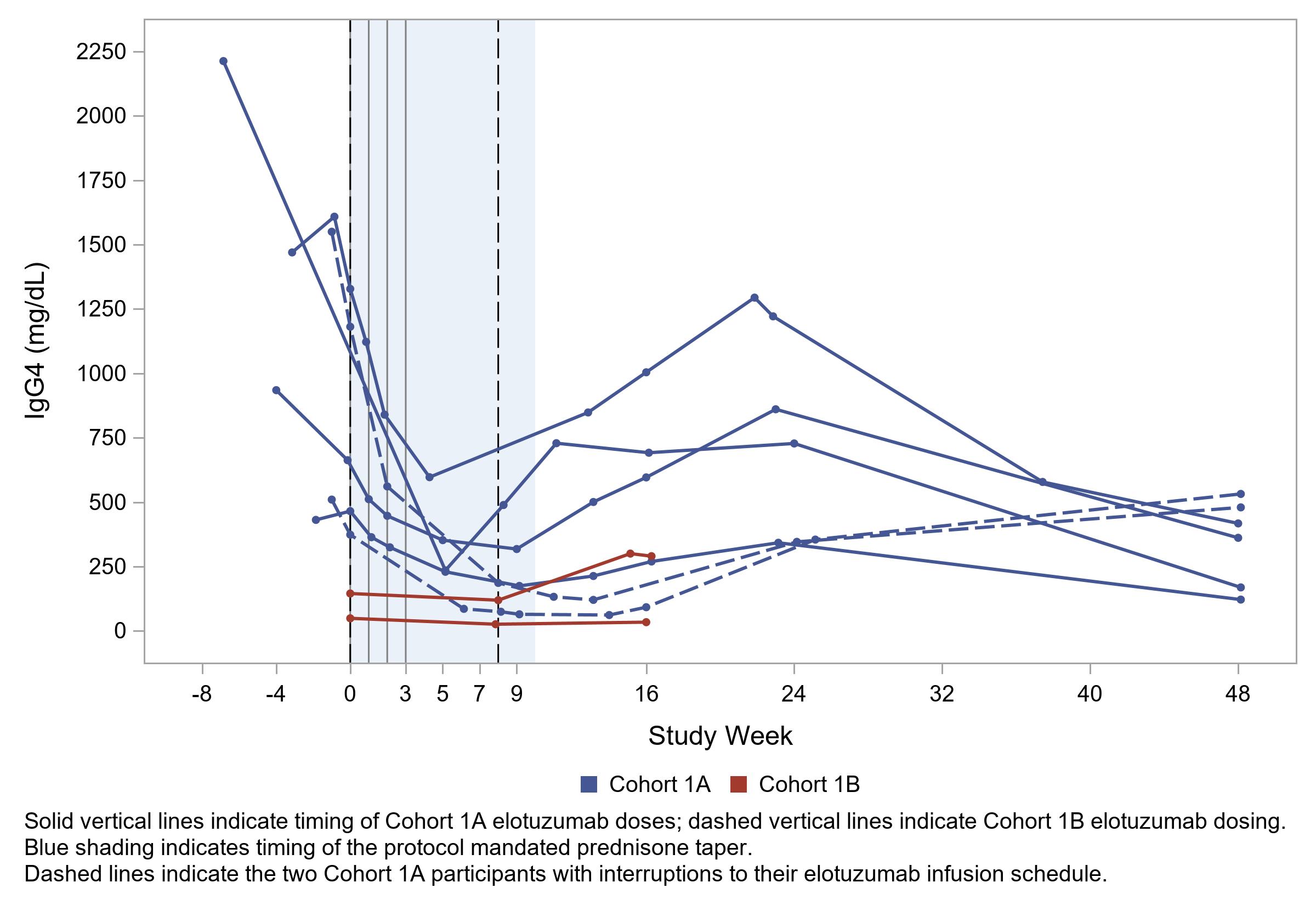
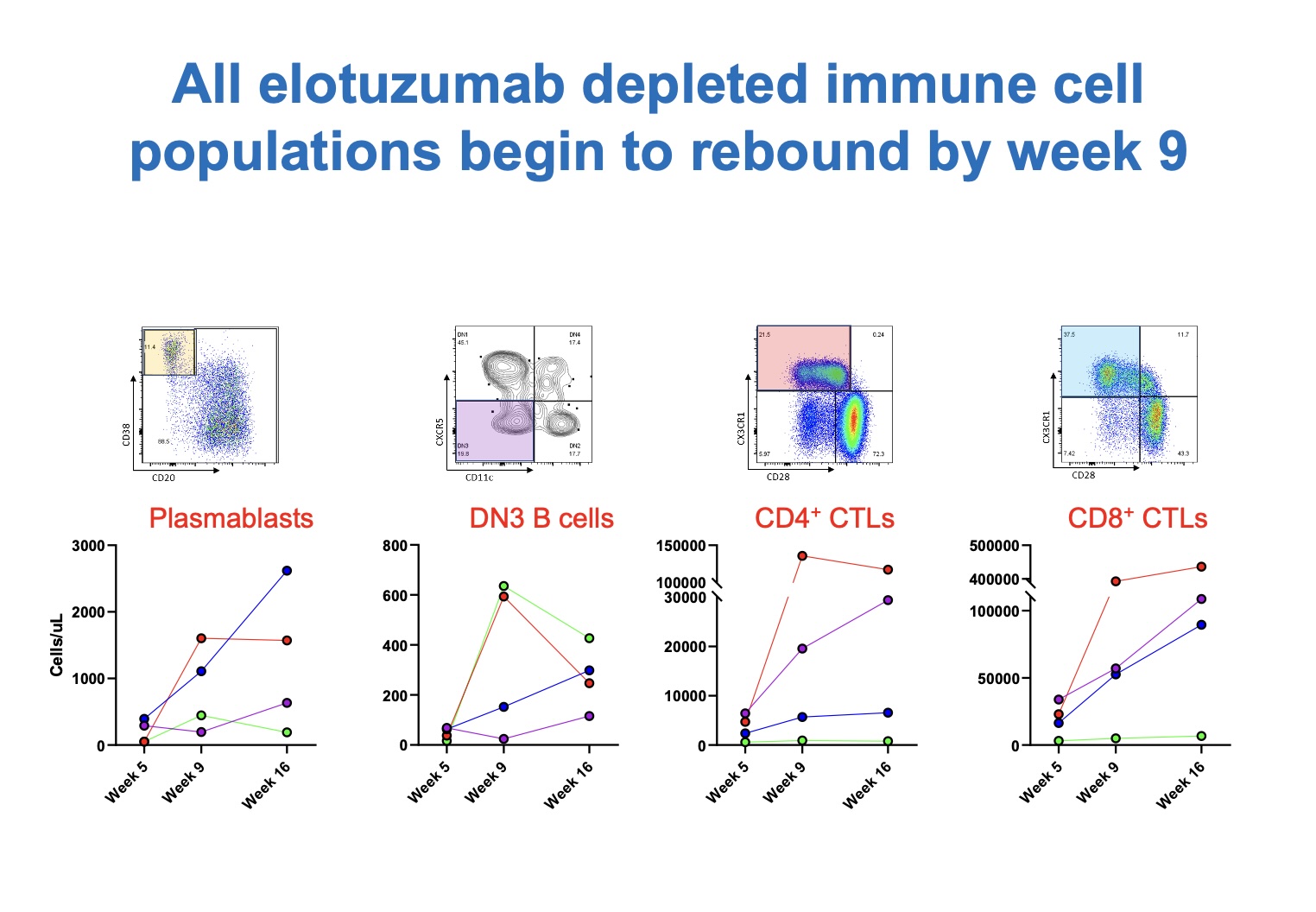
Clinical disease flares were observed in 4 of the first 6 patients at timepoints ranging from 11 weeks to 24 weeks. Serum IgG4 concentrations were observed to rise in all patients (Figure 2) at approximately 16 weeks. In addition, all elotuzumab-depleted immune cell populations (e.g., plasmablasts, DN3 B cells, CD4+ and CD8+ T cells) had begun to rebound by 9 weeks (Figure 3). For these reasons, the protocol was revised such that single doses of elotuzumab would be administered at baseline and then at weeks 8, 16, 24, 32, and 40, with the primary safety endpoint assessed through 48 weeks.
In the second cohort, both patients enrolled experienced disease flares around the Week 16 visit. The substantial evidence of the lack of any sustained efficacy in both cohorts led to the investigators’ decision to terminate the trial.
Conclusion: Elotuzumab does not appear to be an effective treatment approach to the induction and maintenance of remission in IgG4-RD. This appears to be due to its relatively brief depletion effect on the target immune cells that express SLAMF7.
To cite this abstract in AMA style:
Stone J, Khosroshahi A, Majumder S, Perugino C, Fernandes A, McMahon G, Jha I, Varghese J, Delgado A, Katz G, Wallace Z, Pillai S, Barry W, Pinckney A, Goldmuntz E, Welch B, McShea K. Treatment of IgG4-Related Disease (IgG4-RD) with Elotuzumab, an Inhibitor of SLAM-F7: Report of a Phase 2 Clinical Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/treatment-of-igg4-related-disease-igg4-rd-with-elotuzumab-an-inhibitor-of-slam-f7-report-of-a-phase-2-clinical-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-of-igg4-related-disease-igg4-rd-with-elotuzumab-an-inhibitor-of-slam-f7-report-of-a-phase-2-clinical-trial/