Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Giant cell arteritis (GCA) is an inflammatory condition of medium- and large-sized arteries. Prospective clinical trials have demonstrated the efficacy of tocilizumab (TCZ) for treatment of patients with GCA. However, there is a limited data on the use of TCZ in routine clinical practice. The objective of the study was to evaluate the efficacy and safety of TCZ in a retrospective cohort study of patients with GCA treated with TCZ.
Methods: Patients with GCA treated with TCZ at 4 clinical centers of a single tertiary care institution (2000-2020) were identified. The diagnosis of GCA was confirmed by arterial biopsy, large vessel imaging, or clinical diagnosis meeting ACR classification criteria and physician diagnosis. Patient demographics, clinical presentation, laboratory studies, treatment course and adverse events were abstracted from the medical record; only patients with at least 6 months of follow-up after TCZ initiation were included. Kaplan-Meier methods were used to estimate time to TCZ discontinuation and first relapse after discontinuation. Poisson regression models were used to compare relapse rates before and after TCZ initiation.
Results: The study included 119 patients [61% female; mean(SD) age at GCA diagnosis 70.3(8.2) years]. The majority of patients (89%) had a biopsy-proven and/or imaging-based diagnosis of GCA, while 13(11%) had a clinical diagnosis of GCA. In addition to glucocorticoids, 40(34%) patients received other immunosuppressive agents prior to TCZ. The method of initial TCZ administration was subcutaneous (162mg/ml) weekly in 48(41%), subcutaneous every other week in 20(17%), monthly 4mg/kg infusions in 34(29%), monthly 8mg/kg infusions in 14(12%) and non-standard dosing in 3 remaining patients. The median(IQR) duration from GCA diagnosis to TCZ initiation was 4.8(1.2-22.0) months. The median(IQR) duration of TCZ treatment was 18(11-28) months. The mean(SD) dose of prednisone at TCZ initiation was 31(19) mg/day and was reduced to a mean(SD) dose of 3.8(6.6) mg/day at TCZ discontinuation/last follow-up visit. The relapse rate per year decreased 43% from 0.77 to 0.44 after the initiation of TCZ (RR=0.57; 95% CI: 0.44-0.75; p< 0.001). The mean(SD) ESR and CRP decreased from 22(20) mm/hour to 6(9.2) mm/hour and from 19.1(25) mg/L to 5.4(16.6) mg/L, respectively from TCZ initiation to TCZ discontinuation/last follow-up visit. At 2 years of follow-up, 67% of patients had discontinued glucocorticoids. At last follow up, 50 patients had discontinued TCZ, only 16 of which were due to adverse events. The median time to TCZ discontinuation was 2.4 years. The most common adverse events were infections and cytopenias. While on TCZ, 1 patient developed new onset vision loss related to GCA and 1 patient, without history of diverticulitis, had bowel perforation. Among those discontinuing TCZ, 61% had relapsed at least once by 1 year after discontinuation.
Conclusion: TCZ use significantly reduced relapse rate and prednisone dosage. Patients tolerated long-term use with only 13% discontinuing due to adverse events. However, the high relapse rate after discontinuation indicates that ongoing use may be required beyond two years to maintain remission.
 Image 1: Time from TCZ start to TCZ discontinuation
Image 1: Time from TCZ start to TCZ discontinuation
 Image 2: Time to first relapse after TCZ stop
Image 2: Time to first relapse after TCZ stop
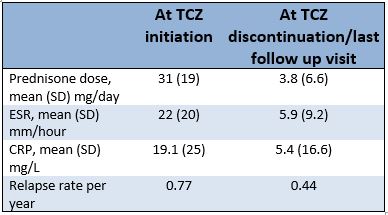 Table 1: Main outcome variables
Table 1: Main outcome variables
To cite this abstract in AMA style:
rakholiya j, Koster M, Langenfeld H, Crowson C, Abril A, Bansal P, Mertz L, Rodriguez Pla A, Sehgal R, Wang B, Warrington K. Treatment of Giant Cell Arteritis with Tocilizumab: A Retrospective Cohort Study of 119 Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/treatment-of-giant-cell-arteritis-with-tocilizumab-a-retrospective-cohort-study-of-119-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-of-giant-cell-arteritis-with-tocilizumab-a-retrospective-cohort-study-of-119-patients/
