Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Treatment adherence is essential for achieving sustained disease control and improving outcomes in patients with ankylosing spondylitis (AS). This study aimed to evaluate real-world drug survival and treatment adherence among patients with AS treated with tumor necrosis factor inhibitors (TNFi), interleukin-17 inhibitors (IL-17i), or Janus kinase inhibitors (JAKi).
Methods: We conducted a retrospective cohort study using a de-identified, federated electronic health record database (TriNetX Research Network, Cambridge, MA; Date of access: May 28, 2024), which includes data from 89 healthcare organizations and over 125 million patients. We included adult patients (≥18 years old) with a diagnosis of AS; ICD-10: M45.9) between 2004 and 2024 who were initially treated with a tumor necrosis factor inhibitor (TNFi), interleukin-17 inhibitor (IL-17i), or Janus kinase inhibitor (JAKi) for at least 90 days following their AS diagnosis. Patients receiving combination therapy (i.e., multiple medication classes initiated on the same day) or who did not meet the 90-day treatment duration threshold were excluded. The primary outcome was medication non-adherence, defined as a switch to a different medication class within 180 days of the initial prescription date.To address baseline differences across treatment groups, we performed 1:1 greedy nearest-neighbor propensity score matching with a caliper = 0.1, conducted separately for the TNFi vs. IL-17i and TNFi vs. JAKi comparisons. Matching covariates included age at index, race, ethnicity, and additional comorbidities (see Table 1). Logistic regression models were used to assess the association between therapeutic class and non-adherence, with results presented as odds ratios (ORs) and 95% confidence intervals (CIs). Time to treatment withdrawal (TPTW) was evaluated using Cox proportional hazards regression, with results presented as hazard ratios (HR) and 95% confidence intervals. The results were visualized using Kaplan-Meier Curves. Results are reported both before and after propensity score matching.
Results: The study included patients with AS, 12,180 were initiated on TNFi, 1,211 on IL-17i, and 327 on JAKi. After propensity matching for age, sex, race, ethnicity and co-morbidities, the groups were well balanced (Table 1). Non-adherence risk was seen in 2.6% on TNFi compared to 7.1% on IL-17i and 3.1% on TNFi compared to 9.2% on JAKi. Non-adherence odds were significantly higher in patients initially prescribed IL-17i before and after matching (OR= 2.85, 95% CI:1.87, 4.4.47) than in patients started on TNFi. Similarly, the odds of non-adherence were higher in patients with JAKi before and after matching (OR=3.20, 95% CI: 1.59, 7.00) than in patients on anti-TNF (Table 2). Kaplan-Meier analysis (Figure 1) demonstrated higher treatment adherence and drug survival among TNFi users, compared to both IL-17i and JAKi (Figure 1).
Conclusion: In this large real-world cohort of AS patients, initial treatment with TNFi was associated with significantly better adherence and drug survival compared to IL-17i and JAKi therapies. These findings may inform therapeutic decision-making and highlight the importance of initial treatment choice in AS management.
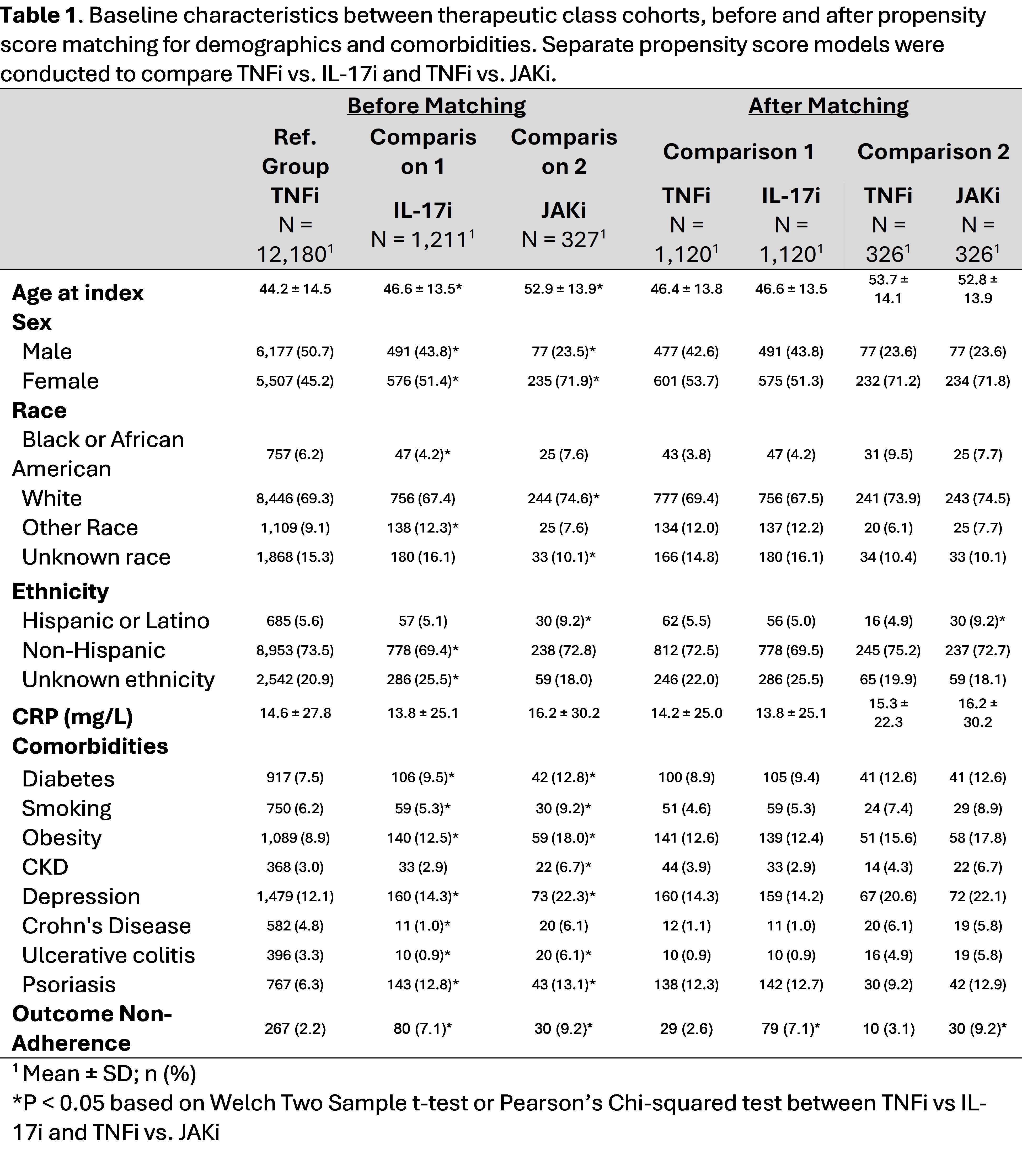 Table 1. Baseline characteristics between therapeutic class cohorts, before and after propensity score matching for demographics and comorbidities. Separate propensity score models were conducted to compare TNFi vs. IL-17i and TNFi vs. JAKi.
Table 1. Baseline characteristics between therapeutic class cohorts, before and after propensity score matching for demographics and comorbidities. Separate propensity score models were conducted to compare TNFi vs. IL-17i and TNFi vs. JAKi.
.jpg) Table 2. Odds ratios (95% Confidence interval) and Hazard Ratios (95% Confidence interval) of medication non-adherence as a function of initial therapeutic class, before and after propensity score matching for demographics and clinical characteristics.
Table 2. Odds ratios (95% Confidence interval) and Hazard Ratios (95% Confidence interval) of medication non-adherence as a function of initial therapeutic class, before and after propensity score matching for demographics and clinical characteristics.
.jpg) Figure 1. Kaplan-Meier survival curve for medication adherence probability 90-180 days following initial prescription date by TNFi vs. IL-17i (left panel) and TNFi vs. JAKi (right panel), after propensity score matching for demographics and comorbidities.
Figure 1. Kaplan-Meier survival curve for medication adherence probability 90-180 days following initial prescription date by TNFi vs. IL-17i (left panel) and TNFi vs. JAKi (right panel), after propensity score matching for demographics and comorbidities.
To cite this abstract in AMA style:
Magrey M, Murphy J. Treatment adherence in ankylosing spondylitis: A comparison of TNFi, IL-17i, and JAKi using real-world data [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/treatment-adherence-in-ankylosing-spondylitis-a-comparison-of-tnfi-il-17i-and-jaki-using-real-world-data/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-adherence-in-ankylosing-spondylitis-a-comparison-of-tnfi-il-17i-and-jaki-using-real-world-data/
