Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: American College of
Rheumatology (ACR) guidelines recommend lowering serum urate (sUA) to a target value
in patients with gout to prevent crystal deposition/promote crystal dissolution.
At a minimum, sUA should be reduced to <6 mg/dL;
in patients with tophaceous deposits/greater disease severity, the sUA target may
be <5. sUA measurement is a necessary step in the treat to target
paradigm, but little is known about sUA measurement practices in usual care or
attainment of target sUA. The purpose of this work was to characterize sUA measurement
and sUA target attainment in patients with incident gout in usual ambulatory
care settings in the United States.
Methods: This retrospective cohort study was conducted at 3 geographically,
demographically, and socioeconomically diverse sites of an integrated
healthcare delivery system. The source population included adults enrolled in
the health plan 2001-2010. Patients with >=1 coded gout diagnosis were
identified; patients with >=2 years enrollment prior to first diagnosis and
with no urate-lowering medication or colchicine dispensings prior to diagnosis
were considered incident cases. The participating sites have similarly
formatted administrative, pharmacy, lab results, and electronic health records
databases that enabled collection of demographic, clinical, medication, and
sUA data for each patient from cohort entry through 2013 or until the patient
was censored from the cohort, whichever occurred first. Descriptive statistics
were used to characterize the cohort and to examine sUA measurement and values
at baseline and over time.
Results: The cohort included 72,803 patients, mean (SD) age 60.3 (14.6);
30% female; 15% Hispanic; 57% white, 13% black, 10% Asian; 38% (n=27,780) were
prescribed urate-lowering medication after diagnosis. Across mean study
follow-up of 5.5 (3.1) years, 87% (n=63,366)
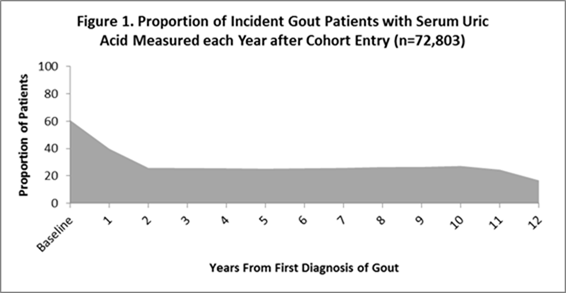
had one or more sUA assessments. The proportion of patients with sUA measured
each year after diagnosis is shown in Figure 1. The average number of sUA
measurements was 3.4 (4.4) per patient, with a mean of 1.5 (1.4) years between
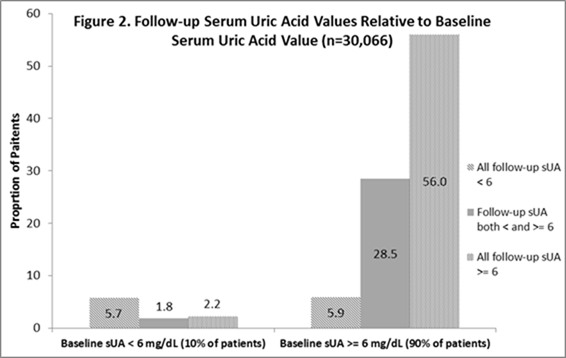
measurements. Only 20% (n=12,491) of patients achieved mean sUA <6. As shown
in Figure 2, 56% (16,827 of 30,066 with >=2 sUA) of patients never had any
sUA <6.
Conclusion: Fully
80% of patients with incident gout in these usual care settings in the United
States did not achieve target mean sUA <6 mg/dL. Although most patients had at
least one sUA assessment, sUA assessment was infrequent. Given that most
patients with incident gout do not reach target sUA, more frequent sUA measurement
is urged to enable treating to target.
To cite this abstract in AMA style:
Raebel M, Reifler L, Tabano D, Goddard K, Sterrett A, Cheetham TC, Harrold L, Sapp D, Schmidt M, Nuevo J, Morlock R, Nichols G. Treating to Target in Gout: The Epidemiology of Serum Urate Measurement Among Patients with Incident Gout in Usual Care Settings in the United States [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/treating-to-target-in-gout-the-epidemiology-of-serum-urate-measurement-among-patients-with-incident-gout-in-usual-care-settings-in-the-united-states/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treating-to-target-in-gout-the-epidemiology-of-serum-urate-measurement-among-patients-with-incident-gout-in-usual-care-settings-in-the-united-states/