Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: APS ACTION Registry aims to study the course of disease in antiphospholipid antibody (aPL)-positive patients. Although transient ischemic attack (TIA) can develop in aPL-positive patients, the ascertainment of TIA can be challenging due to the need to exclude other conditions, e.g., complex migraine with aura or seizure. Our primary objective was to analyze the clinical characteristics of persistently aPL-positive patients who were reported to have had “TIA” prior to recruitment and/or during prospective follow-up.
Methods: The registry inclusion criteria are positive aPL based on the Updated Sapporo APS Classification Criteria within one year prior to enrollment. Patients are followed every 12±3m with clinical data and blood collection. Firstly, we retrospectively compared the baseline characteristics of patients with a history of TIA among those without or with a history of imaging-confirmed thrombosis (at any time prior to registry entry). Secondly, in patients who completed 1-10 year follow up, we prospectively analyzed those with new onset TIA, which was defined as “a transient episode of neurologic dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction lasting less than 24 hours” (confirmation status by a neurologist is also recorded).
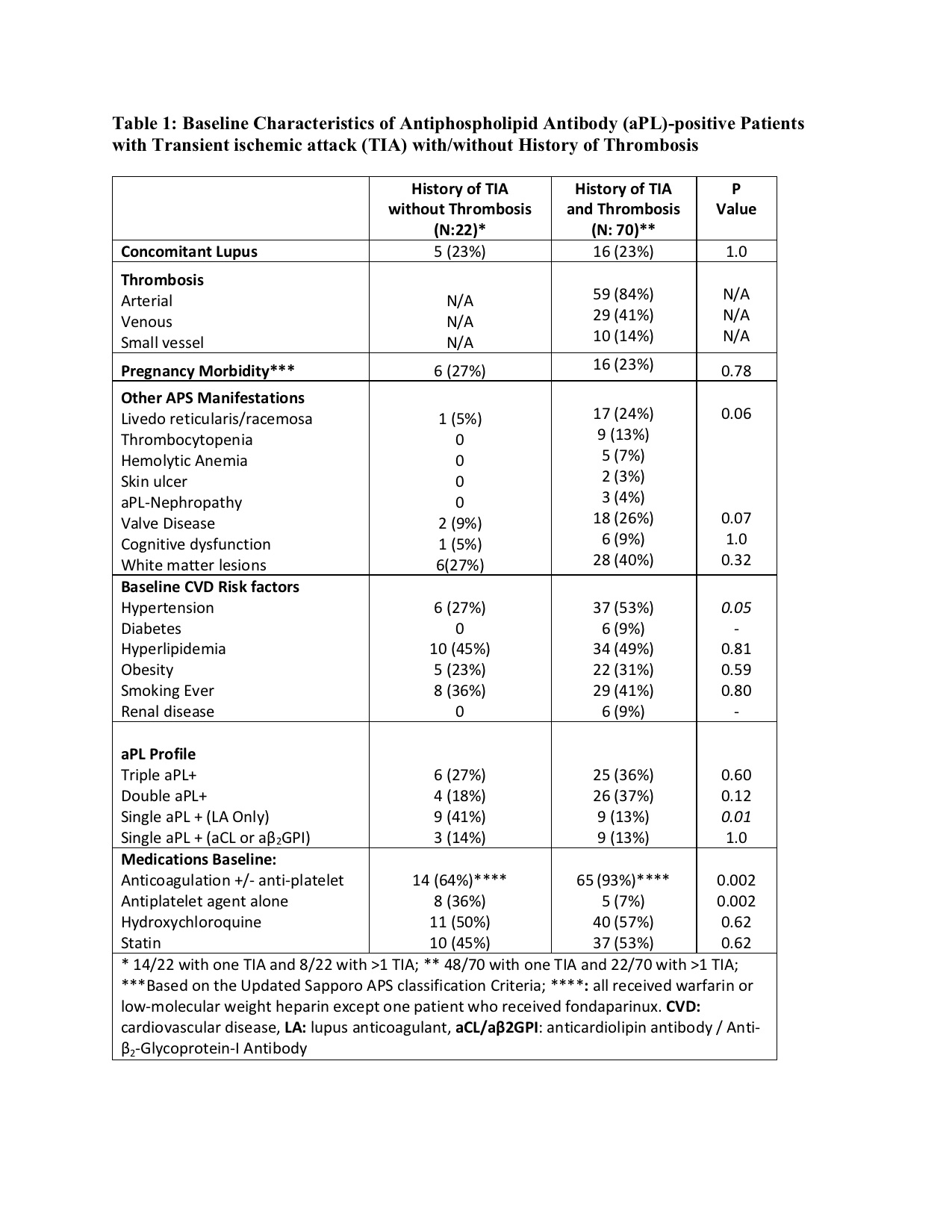
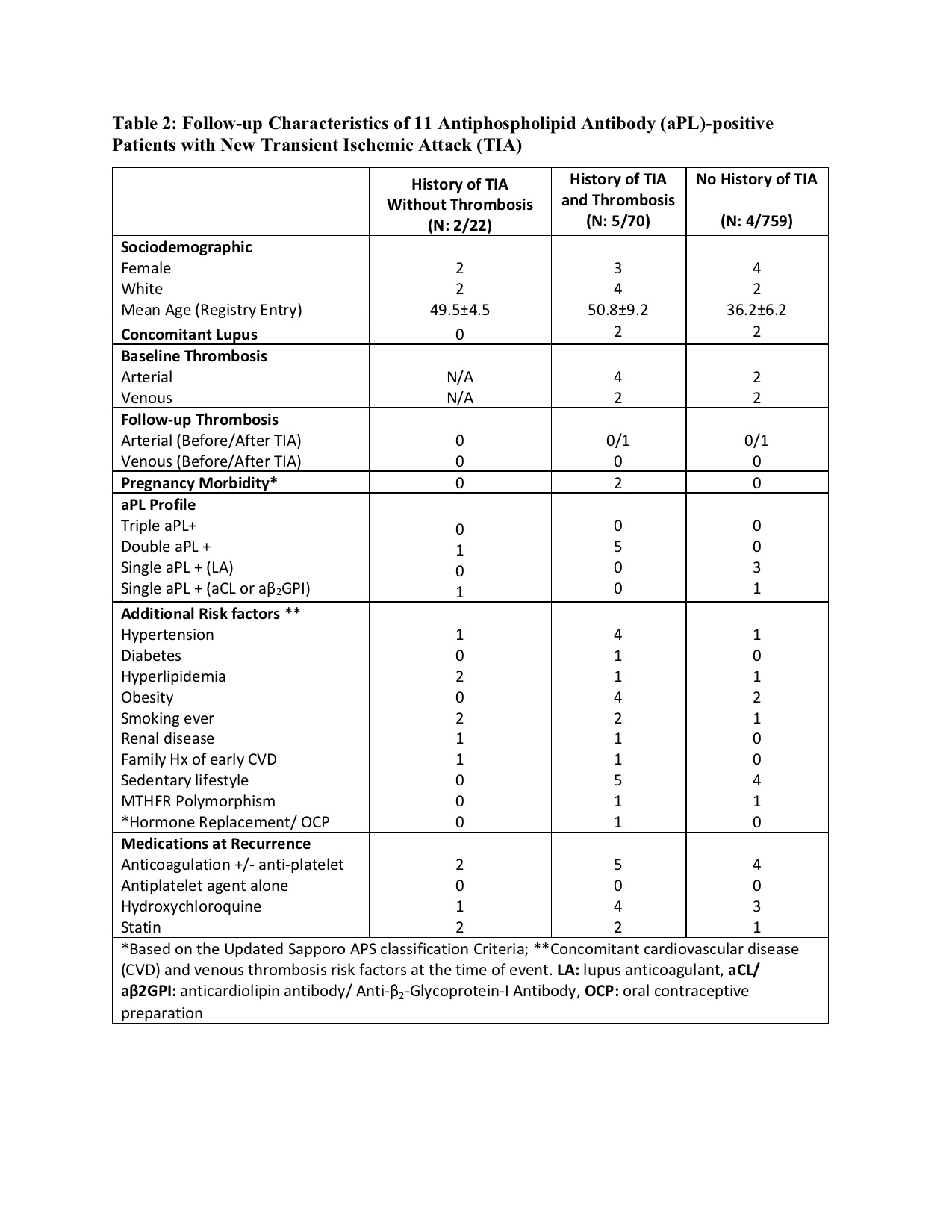
Results: As of April 2023, 1,166 patients were included in the registry and 92 (8%) had a history of TIA at enrollment: 22/92 (24%) without and 70/92 (76%) with history of imaging confirmed arterial/venous thrombosis. Of 22 TIA patients without history of thrombosis (mean age 54.8 ± 11, female 17 [77%], White: 20 [91%]), 15 (68%) were confirmed by a neurologist. Of 70 TIA patients with history of imaging-confirmed thrombosis (mean age 56.7 ± 11, female 39 [56%], White: 48 [69%], arterial thrombosis 59 [84%], and venous thrombosis 29 [41%]), 46 [66%] were confirmed by a neurologist. At baseline, TIA patients without thrombosis were more likely to receive antiplatelet agent alone and have single LA positivity, whereas TIA patients with history of imaging-confirmed thrombosis were more likely to have hypertension and receive warfarin (Table 1). During the prospective follow up of 92 patients with a history of TIA (mean follow-up: 4.5 ± 3.0 years), seven new TIA were reported: a) two (9%) in 22 patients with no history of thrombosis (1.41 per 100 patient-years), and b) five (7%) in 70 patients with history of thrombosis (2.71 per 100 patient-years) (Table 2). In addition, four of 759 (0.5%) patients with no history of TIA at baseline and who completed at least one year follow-up developed a TIA during the mean follow-up of 5.12 years (0.09 per 100-patient years).
Conclusion: In our large international cohort of persistently aPL-positive patients, 8% were reported to have history of “TIA” at the registry entry. The TIA recurrence rate during follow-up was 7-8%; however, the new TIA rate was less than one percent. Approximately two-thirds of patients without a history of imaging-confirmed thrombosis were treated with anticoagulation; all patients with recurrent/new TIA during the follow-up were on anticoagulation. Our findings underscore the need for well-designed controlled studies of TIA in aPL-positive patients with strict definitions.
To cite this abstract in AMA style:
Erton Z, Thaler J, Andrade D, Barber M, Tektonidou M, Sciascia S, Pengo V, Pardos-Gea J, Ruiz-Irastorza G, Lopez-Pedrera C, Belmont H, Nina K, Fortin P, Zuily S, Chighizola C, Signorelli F, Zhang Z, Atsuma T, Efthymiou M, Branch D, Nalli C, Rodriguez almaraz E, Petri M, Pazzola G, Cervera R, Artim Esen B, Shi H, Zuo Y, Quintana R, Willis R, Duarte-Garcia A, Bertolaccini M, Cohen H, Erkan D, Of APS ACTION O. Transient Ischemic Attack in Antiphospholipid Antibody-positive Patients: Retrospective and Prospective Results from the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (“Registry”) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/transient-ischemic-attack-in-antiphospholipid-antibody-positive-patients-retrospective-and-prospective-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networ/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/transient-ischemic-attack-in-antiphospholipid-antibody-positive-patients-retrospective-and-prospective-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networ/