Session Information
Date: Monday, October 27, 2025
Title: (1221–1247) Pain in Rheumatic Disease Including Fibromyalgia Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Evidence-based behavioral treatments for chronic pain are largely inaccessible, particularly to rural patients. This decentralized randomized controlled trial compared the effectiveness of two digital, evidence-based chronic pain management programs—3D immersive Skills-Based Virtual Reality (EaseVRx+) and interactive pain coping skills training (painTRAINER)—in a rheumatic and musculoskeletal disease (RMD) and chronic pain population.
Methods: Patients were recruited from 3 large health centers and practice networks (Cedars-Sinai Medical Center; the University of Alabama and its affiliated Excellence Network in RheumatoloGY to Innovate Care and High-impact research; Ochsner Health System). Patients meeting study inclusion criteria (qualifying diagnostic code in medical record, primary residence ZIP code defined as rural, aged ≥13, ≥4 pain on 0-10 scale, without history of seizure, etc.) were identified within each participating network. Study coordinators screened individuals by phone; eligible and interested individuals were consented electronically. Participants were required to complete 7 daily baseline surveys which recorded demographics, daily pain intensity as measured by an 11-point pain numeric rating scale (NRS), and daily medication use prior to 1:1 randomization. Participants completed 16 REDCap surveys collecting pain intensity, medication use, and other PROs over 12 weeks. We measured the improvement in pain intensity between baseline and week 8, defined by a minimal clinically important difference (MCID) of 2 points on the standard 11-point numeric rating scale. Secondary outcomes included PROMIS anxiety and pain interference, pain catastrophizing, and pain self-efficacy.
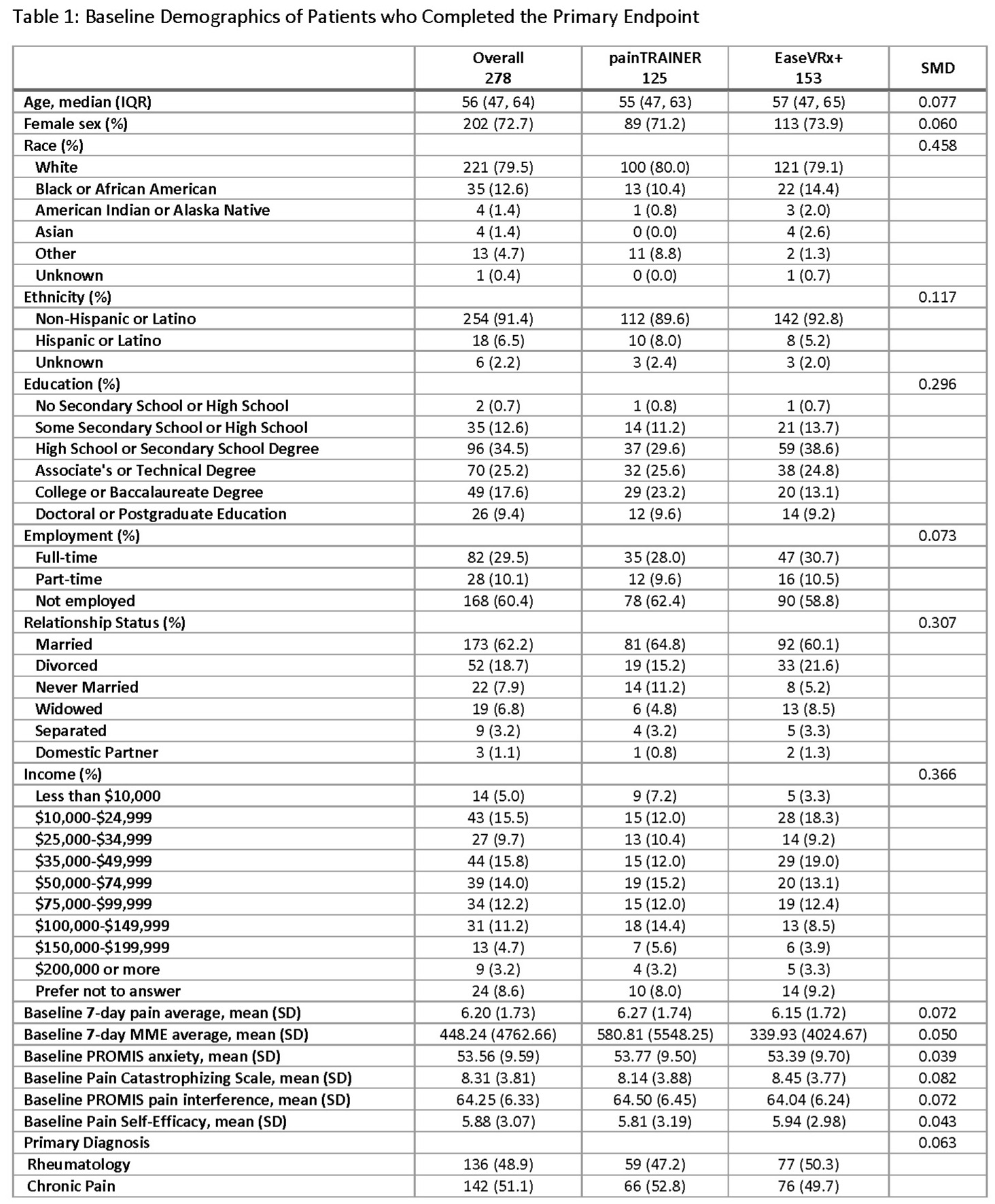
Results: We randomized 330 participants: 169 to EaseVRx+ and 161 to painTRAINER, of which 46.7% were RMD patients. At Week 8, 278 (84.2%) participants completed the primary endpoint, demonstrating a high rate of treatment completion (Table 1). Average pain NRS improved in both arms (mean[SD] 1.22[1.48] units), however the intent-to-treat (ITT) analysis of the primary endpoint found no statistically significant difference in clinically meaningful pain reduction between the EaseVRx+ (22.9% achieving MCID >=2) and painTRAINER arms (32.0%) (p = 0.088) (Figure 1).Between baseline and week 8, the mean (SD) changes in PROMIS pain interference, pain catastrophizing, PROMIS anxiety, and pain self-efficacy for Ease VRx+ and painTRAINER were -3.2 (6.0) vs. -4.5 (6.3), -1.7 (3.2) vs. -1.8 (3.5), -0.6 (7.6) vs. -0.3 (7.6), and 0.6 (2.8) vs. 1.5 (2.7), respectively. The group analyses were not statistically significant, except for pain self-efficacy (greater in the painTRAINER arm at weeks 6, 7 and 8) (Figure 2).
Conclusion: This trial displays the viability of implementing autonomous (self-paced) digital behavioral treatments for RMDs and chronic pain in rural areas, especially for patients without access to in-person behavioral treatment. EaseVRx+ and painTRAINER are effective and provide accessible behavioral pain treatment for chronic pain and could play a significant role in improving health equity in underserved populations.
To cite this abstract in AMA style:
Spiegel B, Curtis J, Eshraghi Y, Guirguis M, Darnall B, Rini C, Holladay E, Muskaan M, Choi S, Eberlein S. Transcending barriers to pain care in rural America: a pragmatic comparative effectiveness trial of evidence-based, on-demand, digital behavioral treatments for chronic pain [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/transcending-barriers-to-pain-care-in-rural-america-a-pragmatic-comparative-effectiveness-trial-of-evidence-based-on-demand-digital-behavioral-treatments-for-chronic-pain/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/transcending-barriers-to-pain-care-in-rural-america-a-pragmatic-comparative-effectiveness-trial-of-evidence-based-on-demand-digital-behavioral-treatments-for-chronic-pain/

.jpg)
.jpg)