Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Hand osteoarthritis (OA) is the most common form of arthritis affecting the hand, but therapeutic options for OA at the distal interphalangeal (DIP) joint have limited efficacy. While the primary outcome of a randomized controlled clinical trial evaluating traction of the DIPs was null, we explored whether secondary outcome measures indicated a benefit of the treatment.
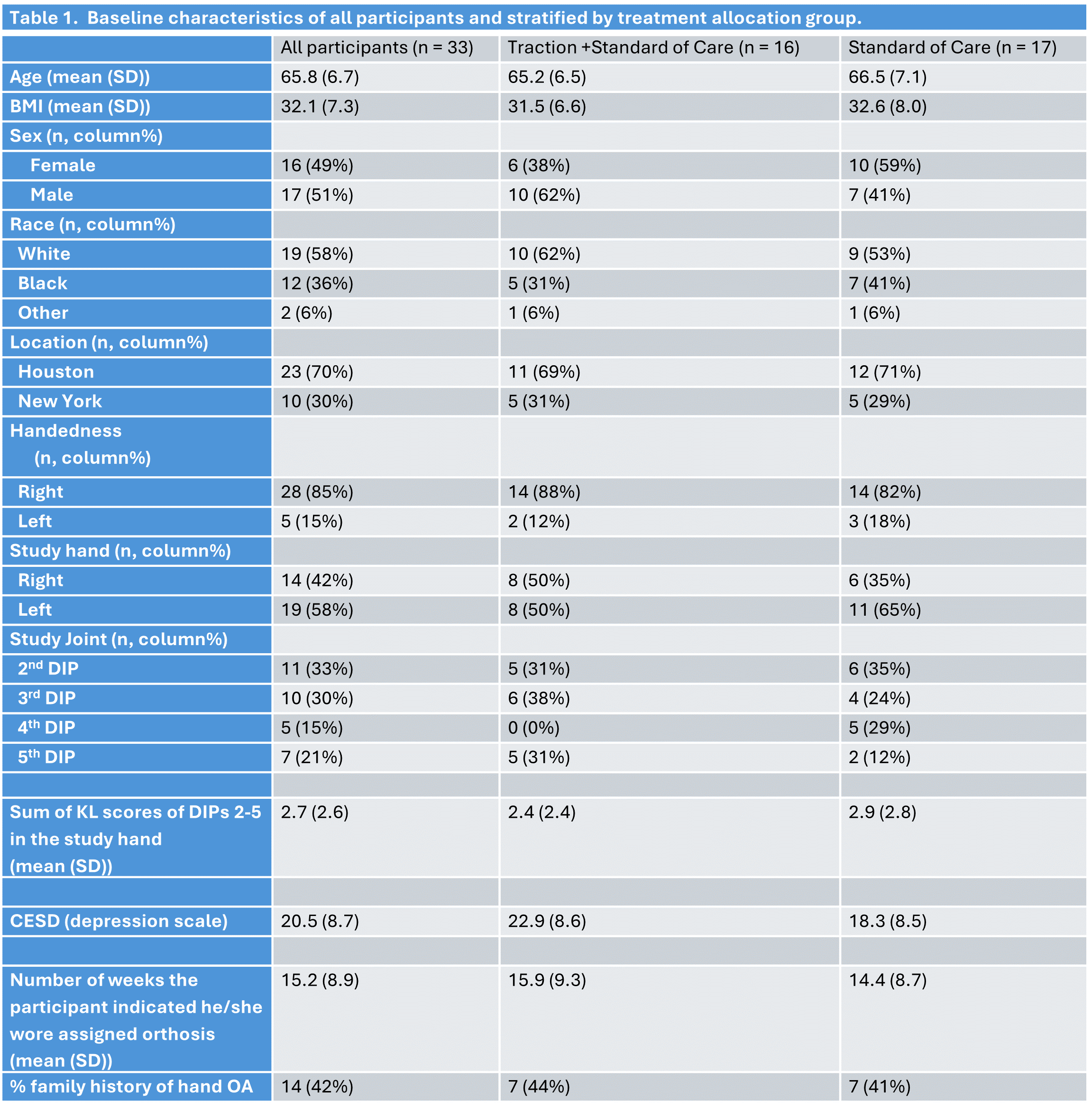
Methods: In this pilot multi-center, double-blind, controlled trial, of DIP hand OA in at least 3 joints, patients were randomized to a traction device (fig 1) along with standard of care vs standard of care. Patients were evaluated at baseline and at weeks 4, 12, and 24 for multiple measures of pain as well as functional assessments using questionnaires (the Disabilities of Arm Shoulder and Hand (DASH), the Michigan Hand Questionnaires, the Functional Index for Hand Osteoarthritis (FIHOA)) and clinical tests including the Functional Dexterity test, grip strength test, pinch strength test, and tenderness on joint palpation. All participants randomized into the study were included. We used a last observation carried forward strategy to address missing data.
Results: 33 participants were enrolled and randomized to either the experimental or control arm. Of the secondary outcome measures that we evaluated, two were statistically significant in when looking at the 24 week timepoints, these included the DASH and the Michigan Hand Outcome ADLs of both hands. The DASH scores were significantly better in the traction arm compared to the control by nearly 11 points (-10.9 (95% CI -21.8 – -0.2)), and the Michigan Hand Outcome ADLs of both hand scores were also better by 12.0 points (12.0 (1.1 – 22.8)). The 4 other functional hand outcomes, FIHOA, grip strength, AUSCAN function, and the Michigan Hand Outcome overall hand scores, did not meet statistical significance but all showed more improvement in the traction arm compared to the control arm. None of the pain outcome measures showed a signal for being more beneficial in either arm.
Conclusion: In our pilot RCT of finger traction for DIP hand OA, based on our secondary outcomes, there is a signal that function may be improved in the treatment arm. Our findings suggest that functional assessments might be a better outcome measure to evaluate treatment benefit for hand OA interventions compared to pain assessments. A larger clinical trial, perhaps with a focus on function instead of pain as an outcome, is warranted.
To cite this abstract in AMA style:
Lo G, Drillock L, Staines K, Strayhorn M, Tse K, Virtanen G, Welsh L, Seu M, Gersh E, Haugen I, Grainger A, Samuels J. Traction Therapy May Improve Hand Function in Those with Distal Interphalangeal Osteoarthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/traction-therapy-may-improve-hand-function-in-those-with-distal-interphalangeal-osteoarthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/traction-therapy-may-improve-hand-function-in-those-with-distal-interphalangeal-osteoarthritis/

.gif)