Session Information
Date: Tuesday, November 12, 2019
Title: 5T092: RA – Treatments IV: Novel Therapy & Predicting Response (2768–2773)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Biologic Disease Modifying Anti-Rheumatic Drugs (bDMARD) tapering is proposed by clinical practice guidelines in rheumatoid arthritis (RA) patients in sustained remission. However, no randomized control trial (RCT) has been implemented to date to answer the question of tapering tocilizumab (TCZ) or abatacept (ABA).
The ToLEDo (Towards the Lowest Efficacious Dose) trial aimed to assess the impact on disease activity of progressive spacing of TCZ or ABA injections in RA patients in sustained remission compared to their maintenance at full dose.
Methods: ToLEDo is a multicenter open-label non-inferiority (NI) RCT conducted in RA patients fulfilling ACR-EULAR 2010 criteria. Patients had to be 1) treated with ABA or TCZ for ≥ 1 year (monotherapy or in combination with csDMARD, corticosteroid allowed at a dose ≤ 5 mg / day), 2) in DAS28ESR remission (DAS28ESR < 2.6) for ≥ 6 months and 3) with no X-ray damage progression in the year before inclusion. They were randomized into 2 arms: TCZ or ABA maintenance at full dose or DAS28-driven progressive injection spacing arm, in which bDMARD IV or SC injections were progressively spaced out every 3 months according to a predetermined 4-step algorithm up to bDMARD discontinuation at step 4. Spacing was reversed to the previous step in case of flare. The primary outcome was the evolution of disease activity according to DAS44 during the 2-year follow-up, analyzed in a linear mixed-effect model. Secondary outcomes were flare and major flare rates (respectively defined as DAS28 > 3.2, and DAS28 >3.2 not recovered at the following visit despite bDMARD escalation at previous step) were also compared between the 2 arms. Analyses were done per protocol (PP) according to a NI hypothesis (NI margin at 0.25 for DAS44 and 0.07 for flare rates).
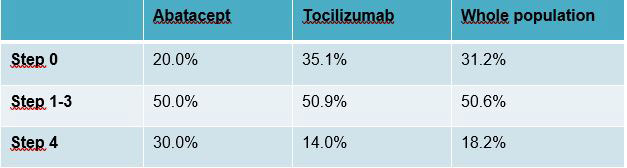
Results: Overall, 233 patients were randomized but 229 were treated and 199 were considered for PP analysis (89 in Spacing arm and 110 in Maintenance arm). 146 (73.4 %) patients were treated with TCZ and 53 (26.6%) with ABA. At the end of the follow-up in the Spacing arm, 18.2% of patients discontinued their bDMARD (step 4), 50.6% had tapered them (step 1 to 3) and 31.2% needed to go back to initial step (step 0) (Table 1). In terms of DAS44, the slope difference was 0.10 [95% CI: -0.10, 0.31] in the whole population, 0.34 [95% CI: -0.07, 0.74] for ABA subgroup and 0.02 [95% CI: -0.22, 0.26] for TCZ subgroup. The upper limit of the 1-sided 95% CI of slope difference exceeded 0.25, failing to demonstrate NI in the whole population and in the ABA subgroup (0.28 and 0.68 respectively), but NI was demonstrated in the TCZ subgroup (0.22, p=0.03) (Figure 1). Flares (Figure 2) were more frequent in the Spacing arm: +0.43 [95% CI: 0.30, 0.55], +0.44 [95% CI: 0.20, 0.68] and +0.42 [95% CI: 0.27, 0.57] in the whole population, ABA and TCZ subgroups respectively. Major flares were more frequent in the Spacing arm: +0.07 [95% CI: -0.01, 0.14], +0.16 [95% CI: -0.05, 0.37] and +0.07 [95%CI: -0.03, 0.16] in the whole population, ABA and TCZ subgroups respectively, compared with Maintenance arm.
Conclusion: The ToLEDo trial failed to demonstrate the NI of the proposed ABA or TCZ tapering strategy in comparison to maintenance at full dose. Thus, it is not in favor of tapering ABA and TCZ according to this scheme.
between Maintenance arm -black- and Spacing arm -red-
To cite this abstract in AMA style:
Kedra J, Dieudé P, Marotte H, Lafourcade A, Ducourau E, Schaeverbeke T, Perdriger A, SOUBRIER M, Morel J, Constantin A, Dernis E, Royant V, Salmon J, Pham T, Gottenberg J, Pertuiset E, Dougados M, Devauchelle Pensec V, Gaudin P, Cormier g, Goupille P, Mariette X, Berenbaum F, Alcaix D, Rouidi S, Berthelot J, Monnier A, Piroth C, Lioté F, Goeb V, Gaujoux-Viala C, Chary-Valckenaere I, Hajage D, Tubach F, Fautrel B. Towards the Lowest Efficacious Dose (ToLEDo): Results of a Multicenter Non-Inferiority Randomized Open-Label Controlled Trial Assessing Tocilizumab or Abatacept Injection Spacing in Rheumatoid Arthritis Patients in Remission [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/towards-the-lowest-efficacious-dose-toledo-results-of-a-multicenter-non-inferiority-randomized-open-label-controlled-trial-assessing-tocilizumab-or-abatacept-injection-spacing-in-rheumatoid-arthrit/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/towards-the-lowest-efficacious-dose-toledo-results-of-a-multicenter-non-inferiority-randomized-open-label-controlled-trial-assessing-tocilizumab-or-abatacept-injection-spacing-in-rheumatoid-arthrit/