Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Clinical practice guidelines for Juvenile Idiopathic Arthritis (JIA) emphasize the importance of adapting guideline recommendations to each individual patient through shared decision-making among patients, families, and clinicians. In the initial treatment of non-systemic JIA, a key decision is whether to start NSAIDs and joint injections alone (NSAIDs+/-JI) or in combination with methotrexate (MTX+NSAIDs+/-JI). Effective shared decision making requires patients and families to be informed of reasonable estimates of the likelihood of side effects (SE) with each treatment option. However, there are limited published prediction models for MTX-associated SE and no models comparing MTX+NSAIDs+/-JI to NSAIDs+/-JI. In this study, we aimed to develop and externally validate a clinical prediction model that provides personalized probabilities of SE comparing NSAIDS+/-JI with and without adding MTX.
Methods: We used data from two Canadian JIA cohorts. Data collected from 2005-2010 in the Research in Arthritis in Canadian Children Emphasizing Outcomes (ReACCh-Out) cohort for model development and data collected from 2017-2020 in the Canadian Alliance of Pediatric Rheumatology Investigators (CAPRI) JIA Registry for external validation. Parents reported SE using standardized lists of symptoms. For model development, we compared various logistic regression models with discrimination accuracy measured with c-index. We measured model calibration by comparing predicted probabilities versus observed rates of SE. Due to the very low frequency of serious SE, models could only predict probabilities for mild-moderate SE. With input from patients, families, and clinicians, we incorporated the models in a Web App that generates a visual aid presenting personalized probabilities of SE when predictor variables are inputted by a clinician.
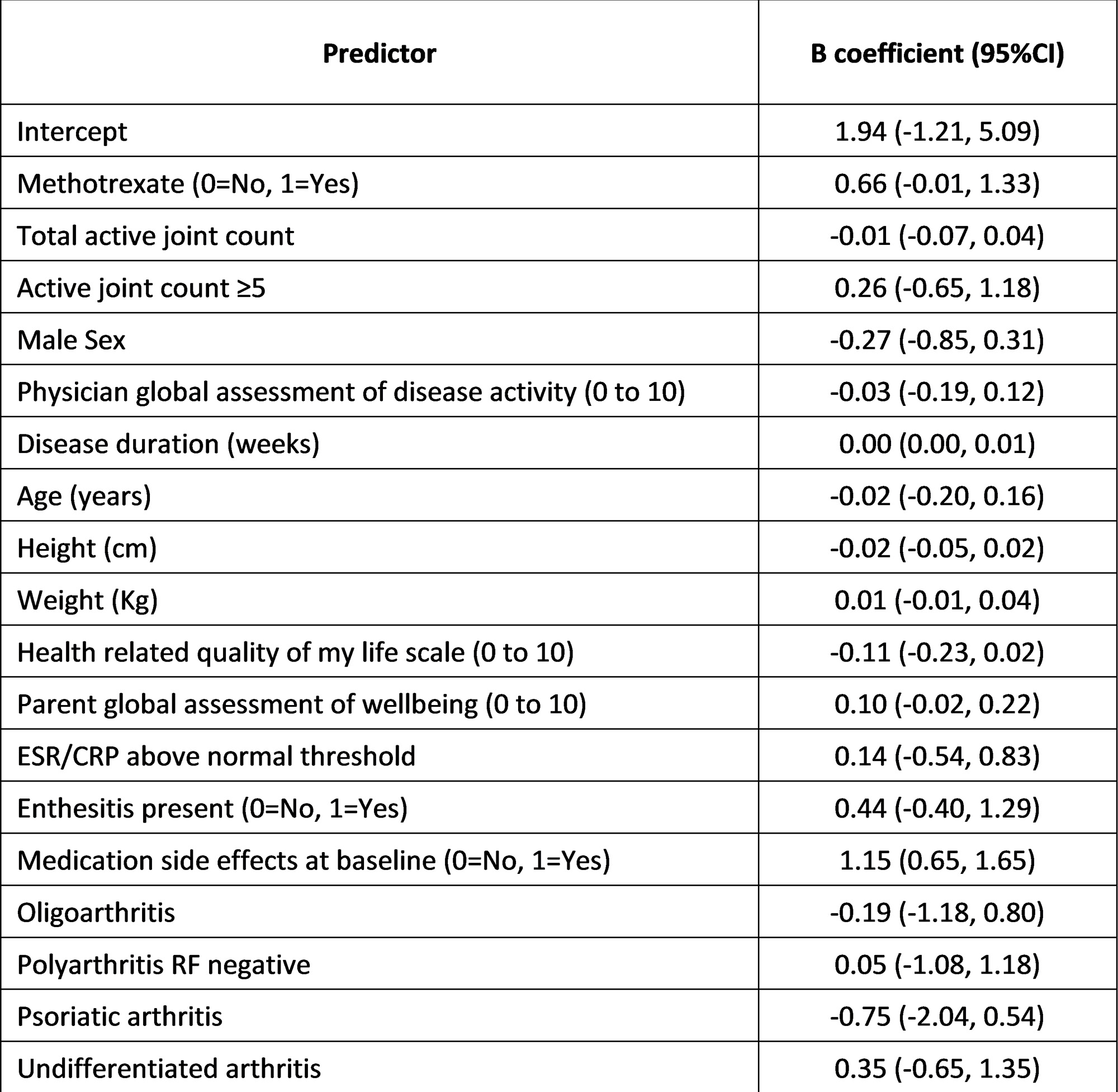
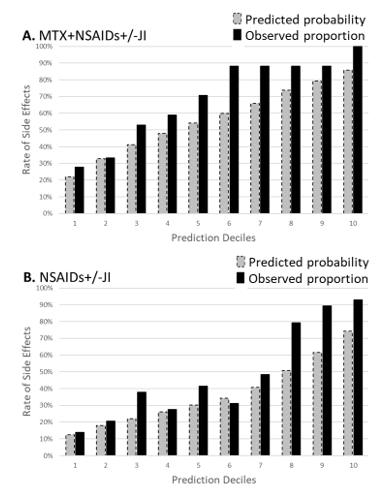
Results: Patients included for development (n=757) and validation (n=437) had baseline characteristics comparable to most North American JIA inception cohorts. Two-thirds % were female with a median age of 8 years old and more than 75% diagnosed with oligoarthritis, RF-negative polyarthritis or enthesitis related arthritis. The c-indices for the various prediction models in the development cohort were all similar at ~0.67. We chose the final model prioritizing fewer and more easily obtainable variables (Table 1). In external validation, our final model for predicting SE had a c-index of 0.80 (95% CI 0.76, 0.84). Model calibration was acceptable, with similar predicted versus observed SE rates (Figure 1). A Web App was created to present probabilities of SE in pie charts, comparing NSAIDs+/-JI to MTX+NSAIDs+/-JI (Figure 2).
Conclusion: This study developed and externally validated a prediction model for probability of SE directly comparing two realistic treatment options at the time of JIA diagnosis. Inputting the variables in a Web app will then produce a visual aid to facilitate shared decision-making with families. Future work should add likelihood of treatment response to further aid the decision of choosing an initial treatment option in JIA.
To cite this abstract in AMA style:
Park J, Loughin T, Henrey A, Guzman J. Towards Effective Shared Decision Making – Development and Validation of a Prediction Model for Personalized Probabilities of Side Effects in the Initial Treatment of Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/towards-effective-shared-decision-making-development-and-validation-of-a-prediction-model-for-personalized-probabilities-of-side-effects-in-the-initial-treatment-of-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/towards-effective-shared-decision-making-development-and-validation-of-a-prediction-model-for-personalized-probabilities-of-side-effects-in-the-initial-treatment-of-juvenile-idiopathic-arthritis/