Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Tofacitinib is a novel, oral Janus kinase (JAK) inhibitor for the treatment of rheumatoid arthritis (RA). Tolerability remains an ill-defined construct in clinical trials. Most commonly the term is used to refer to non-serious adverse events (AEs) that despite being non-serious may still impact the patient’s experience taking a drug, potentially affecting satisfaction and adherence. The objective of this analysis is to describe occurrence of the most common non-serious AEs (excluding infections and laboratory test abnormalities) in patients treated with tofacitinib as monotherapy or in combination with nonbiologic DMARDs.
Methods: The most common non-serious investigator-reported AEs (those with an incidence rate (IR) ≥5 per 100 patient-years and IR exceeding that for patients taking PBO for both doses of tofacitinib combined) were evaluated in a post-hoc pooled analysis of five DMARD inadequate responder Phase 3 studies. IRs were computed for patients taking placebo (PBO) or tofacitinib 5 or 10 mg twice daily (BID) using the data from all five Phase 3 studies. Additionally, the proportion of patients experiencing an AE were computed for each group within the first three months of exposure for the monotherapy study and for the combined four studies in which tofacitinib was taken with nonbiologic DMARDs.
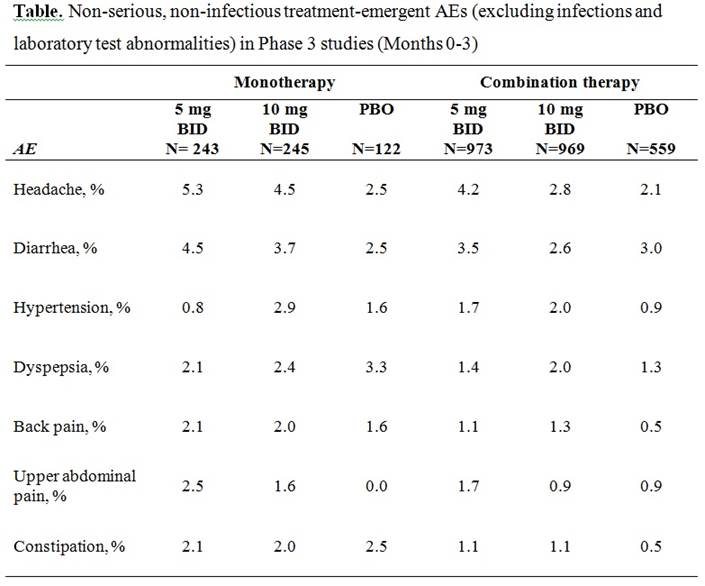
Results: The most commonly reported non-serious AEs (excluding infections and laboratory test abnormalities) were headache, diarrhea, hypertension, dyspepsia, back pain, upper abdominal pain, and constipation. Incidence rates per 100 patient-years for tofacitinib 5 mg BID, 10 mg BID and PBO, respectively, from the pooled Phase 3 data were: headache: 21, 15 and 11; diarrhea: 16, 12, and 10; hypertension: 7, 9, and 4; dyspepsia: 7, 9, and 7; back pain: 6, 7, and 3; upper abdominal pain: 8, 5, and 3; constipation: 6, 6, and 4. The proportion of patients with one or more events in the first three months was <5% for all AEs except headache in patients taking tofacitinib 5 mg BID (see Table).
Conclusion: The most common non-serious AEs (excluding infections and laboratory test abnormalities) were headache, hypertension, back pain and abdominal pain, and selected gastrointestinal events. A rate of 10 or more events per 100 patient-years for at least one dose of tofacitinib was observed for headache and diarrhea. Overall, the proportions of patients experiencing non-serious, non-infectious AEs were similar for patients receiving tofacitinib as monotherapy or in combination with nonbiologic DMARDs.
Disclosure:
A. Dikranian,
Pfizer Inc, Amgen, AbbVie, UCB, Genentech, BMS,
8;
K. Soma,
Pfizer Inc,
1,
Pfizer Inc,
3;
R. Riese,
Pfizer Inc,
1,
Pfizer Inc,
3;
D. Gruben,
Pfizer Inc,
1,
Pfizer Inc,
3;
T. V. Jones,
Pfizer Inc,
1,
Pfizer Inc,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tolerability-and-non-serious-adverse-events-in-rheumatoid-arthritis-patients-treated-with-tofacitinib-as-monotherapy-or-in-combination-therapy/