Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 11:30AM-1:00PM
Background/Purpose: Tofacitinib is an oral JAK inhibitor that is being investigated for the treatment of adult patients (pts) with AS.
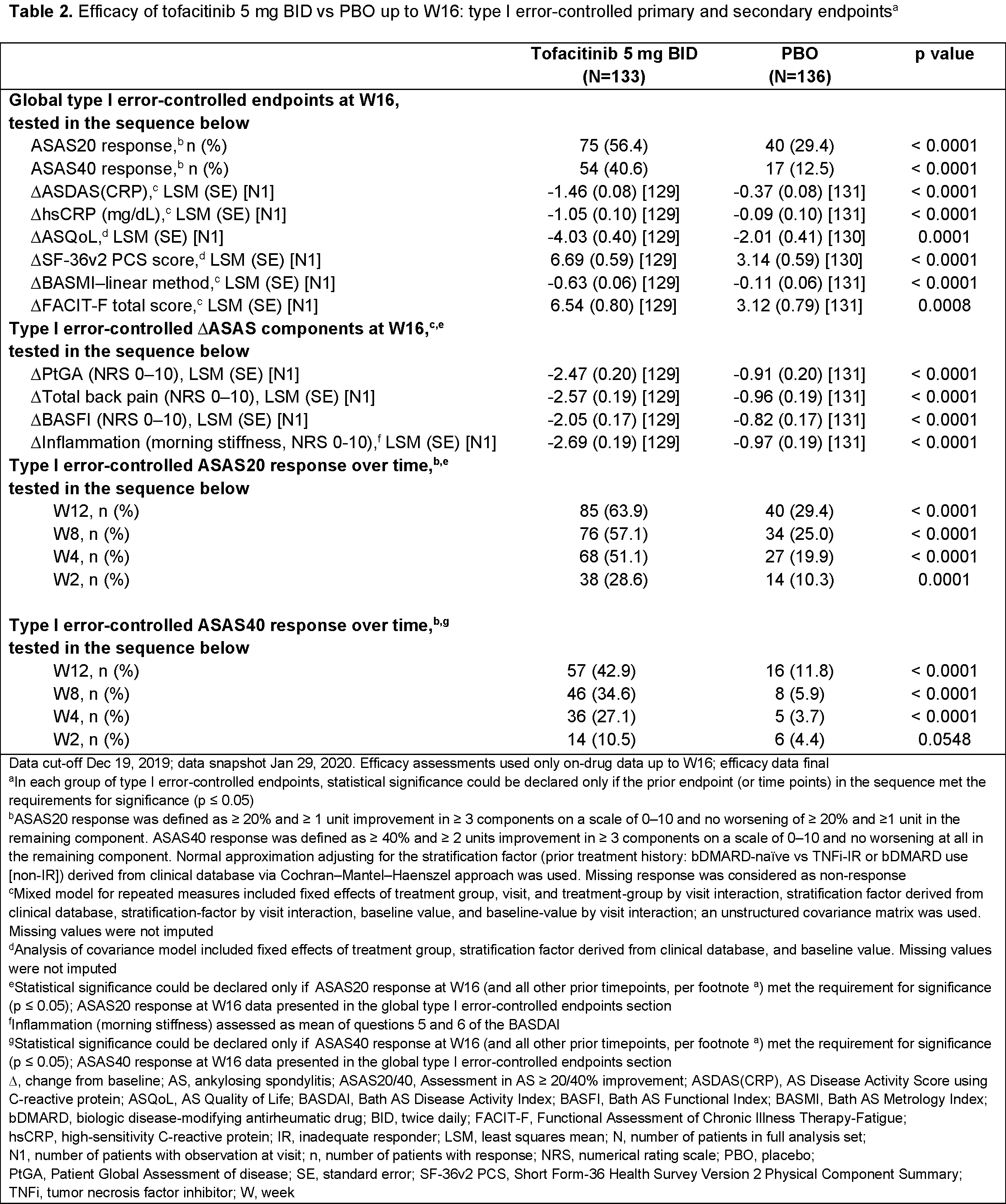
Methods: This Phase 3, randomized, double-blind (DB), placebo (PBO)-controlled study (NCT03502616) enrolled pts aged ≥ 18 years with a diagnosis of active AS documented with centrally read radiographs, who met modified New York Criteria, and had an inadequate response or intolerance to ≥ 2 NSAIDs. In the 16-week DB phase, pts were randomized 1:1 to receive tofacitinib 5 mg BID or PBO. After Week (W)16, all pts received open-label tofacitinib until W48. We report the primary analysis of the study. Four separate families of efficacy endpoints were tested in hierarchical sequences to control for type I error: 1) ASAS20 response at W16 (primary endpoint), ASAS40 response at W16 (key secondary endpoint), change from baseline (∆) to W16 in ASDAS(CRP), hsCRP, ASQoL, SF‑36v2 PCS, BASMI–linear method, and FACIT-F total score; 2) ∆ to W16 in ASAS components: PtGA, total back pain, BASFI, and Inflammation (morning stiffness); 3) ASAS20 response over time; 4) ASAS40 response over time. Statistical tests were conducted at the 2-sided 5% significance level. Safety data up to W16 and up to W48 are presented (data cut-off Dec 19, 2019; database was not locked and study was ongoing).
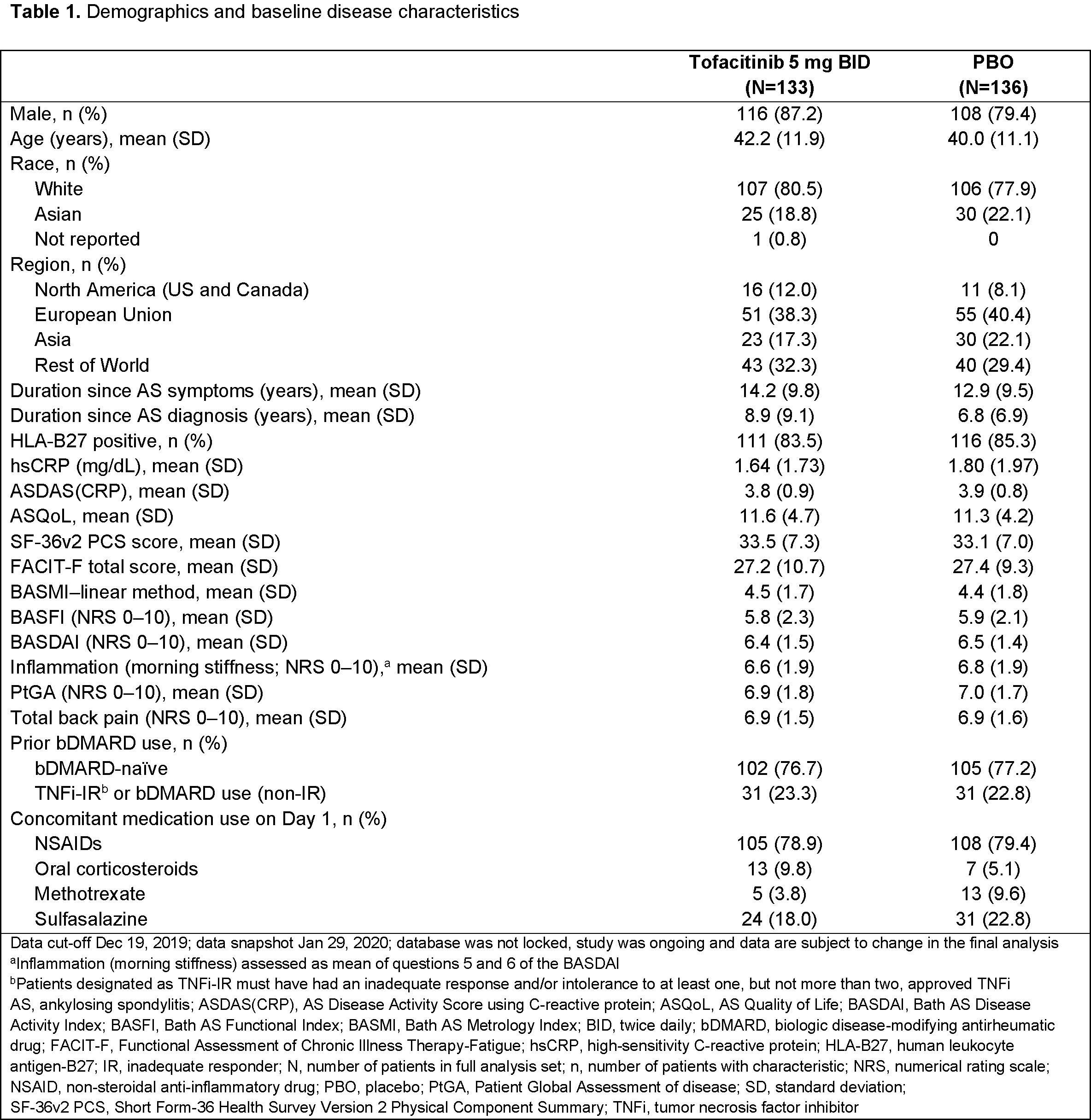
Results: 269 pts were randomized and treated (tofacitinib, n=133; PBO, n=136); 77.0% were bDMARD-naïve (Table 1). Efficacy results are shown in Table 2. At W16, the % of ASAS20 responders was significantly greater with tofacitinib (56.4%) vs PBO (29.4%) (p < 0.0001). The % of ASAS40 responders at W16 was significantly greater with tofacitinib (40.6%) vs PBO (12.5%) (p < 0.0001), and significant improvements with tofacitinib vs PBO were seen for ∆ to W16 in ASDAS(CRP), hsCRP, ASQoL, SF‑36v2 PCS, BASMI–linear method, and FACIT-F total score. Improvements in ASAS components were significantly greater with tofacitinib vs PBO. Significant improvements with tofacitinib vs PBO were seen from W2 (first post-baseline visit) in ASAS20 response and W4 in ASAS40 response. Up to W16, adverse events (AEs) were reported in 72 pts (54.1%) receiving tofacitinib and 70 pts (51.5%) receiving PBO; serious and severe AEs were each reported in 2 pts (1.5%) receiving tofacitinib and no pts receiving PBO; 3 pts (2.3%) receiving tofacitinib and 1 pt (0.7%) receiving PBO discontinued study drug due to AEs. Safety trends were similar up to W48 (Table 3). With tofacitinib: there were no deaths, DVT, PE, ATE, GI perforation, ILD, MACE, malignancies, or opportunistic infections up to W48; adjudicated hepatic events were reported in 1 pt (0.8%) up to W16 and 2 pts (1.5%) up to W48; 1 pt (0.8%) had a serious infection (meningitis) up to W16; 3 pts (2.3%) had non-serious HZ up to W48. With PBO: there were no deaths or AEs of special interest up to W16; 1 pt (0.7%) in the PBO → tofacitinib group had non-serious HZ up to W48.
Conclusion: Pts with active AS had a rapid clinical response to tofacitinib. Efficacy was significantly greater with tofacitinib vs PBO for primary and secondary endpoints. AEs were more frequent with tofacitinib vs PBO; there were no new safety risks.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Sarah Piggott, CMC Connect, and funded by Pfizer Inc.
To cite this abstract in AMA style:
Deodhar A, Sliwinska-Stanczyk P, Xu H, Baraliakos X, Gensler L, Fleishaker D, Wang L, Wu J, Menon S, Wang C, Dina O, Fallon L, Kanik K, van der Heijde D. Tofacitinib for the Treatment of Adult Patients with Ankylosing Spondylitis: Primary Analysis of a Phase 3, Randomized, Double-blind, Placebo-controlled Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/tofacitinib-for-the-treatment-of-adult-patients-with-ankylosing-spondylitis-primary-analysis-of-a-phase-3-randomized-double-blind-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tofacitinib-for-the-treatment-of-adult-patients-with-ankylosing-spondylitis-primary-analysis-of-a-phase-3-randomized-double-blind-placebo-controlled-study/