Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Tofacitinib is a novel, oral Janus kinase inhibitor being investigated as a targeted immunomodulator and disease-modifying therapy for RA. This 24‑month (Mo) Phase 3 study compared efficacy, including inhibition of structural damage, and safety of tofacitinib vs placebo (PBO) in patients (pts) with active RA with inadequate response to methotrexate (MTX). Here we report 24-mo data to assess consistency of efficacy and safety.
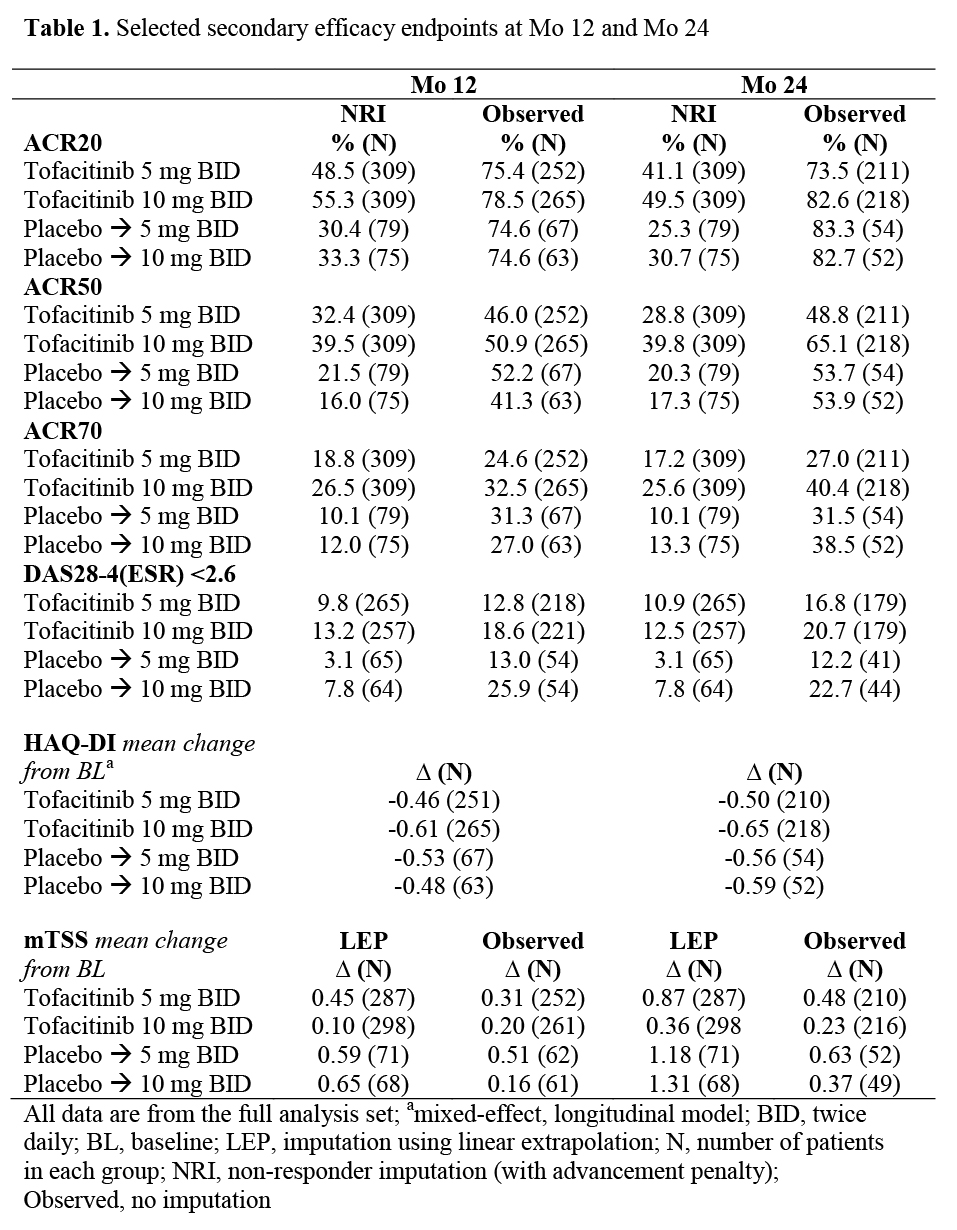
Methods: Pts on stable-dose MTX were randomized 4:4:1:1 to one of four sequences (NCT00847613): tofacitinib 5 mg twice daily (BID); 10 mg BID; PBO advanced to 5 mg BID; PBO advanced to 10 mg BID. Pts on PBO advanced at Mo 6 or at Mo 3 if non‑responsive (<20% reduction from baseline (BL) in swollen and tender joint counts). In the primary analysis, PBO structure data were imputed through linear extrapolation from the time of advancement (Mo 3 or Mo 6). As there is less relevance of imputing data long-term, mean change from BL for the modified Total Sharp Scores (mTSS) as well as ACR response and DAS28-4(ESR) <2.6 rates are shown with and without imputation.
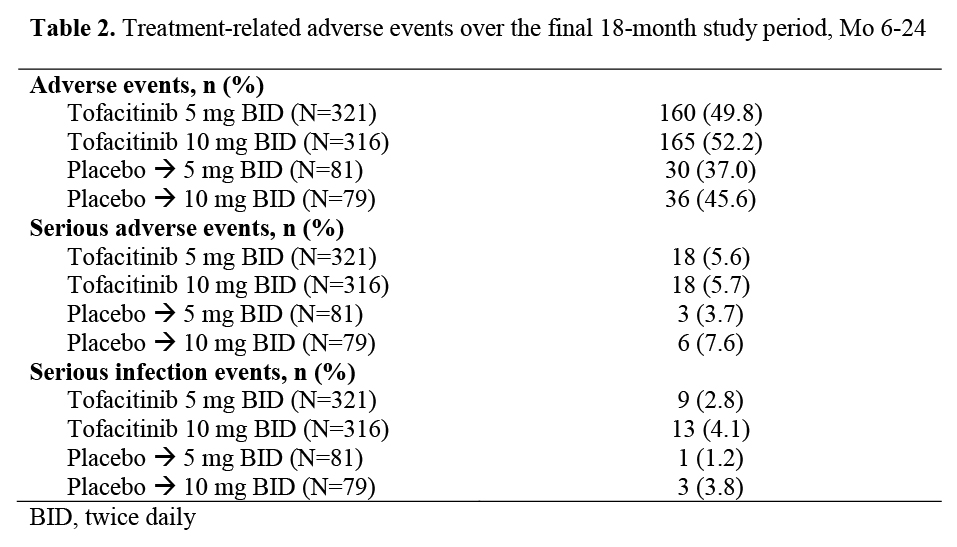
Results: 797 pts were randomized and treated; 535 (67.1%) completed the 24-mo study. Pt treatment sequences were similar for BL characteristics including mTSS and its components. Primary efficacy endpoints and Mo 12 data have been reported previously;1 here we report Mo 24 data. Only descriptive statistics are presented for these selected secondary endpoints (Table 1). Efficacy was maintained through Mo 24 as measured by ACR response, DAS28‑4(ESR) <2.6, HAQ-DI and mTSS suggesting that pts maintain their response to tofacitinib for at least 2 years. Adverse events (AEs), serious AEs and serious infection events are shown in Table 2. Most AEs were mild or moderate and resolved while continuing tofacitinib treatment. During the 24‑mo study, 36 pts (11.2%) on 5 mg BID, 37 pts (11.7%) on 10 mg BID, 8 pts (9.9%) on PBO to 5 mg BID, and 10 pts (12.7%) on PBO to 10 mg withdrew due to AEs related to study drug. There was one opportunistic infection (8 in total over 24 mo) and four deaths (all 5 mg BID; one considered not related: acute myocardial infarction; three considered related by the investigator: cardio‑respiratory arrest; cardiac failure; congestive cardiac and renal failure) occurring after Mo 12. The incidence of laboratory abnormalities was similar in all treatment sequences.
Conclusion: RA pts treated with tofacitinib 5 or 10 mg BID on stable background MTX maintained efficacy, including inhibition of structural damage, through 24 mo. No new safety signals emerged.
Reference:
1. van der Heijde D et al. Arthritis Rheum 2011; 63: S107-S108
Disclosure:
D. van der Heijde,
Abbott Laboratories; Amgen; AstraZeneca; BMS; Centocor: Chugai; Eli-Lilly; GSK; Merck; Novartis; Pfizer; Roche; Sanofi-Aventis; Schering-Plough; UCB; Wyeth,
5,
Imaging Rheumatology,
4;
Y. Tanaka,
Bristol-Myers Squibb; MSD KK; Chugai Pharmaceutical Co Ltd; Mitsubishi-Tanabe Pharma Corporation; Astellas Pharma Inc; Abbott Japan Co, Ltd; Eisai Co, Ltd; Janssen Pharmaceutial KK,
2,
Mitsubishi-Tanabe Pharma Corporation; Abbott Japan Co, Ltd; Eisai Co. Ltd; Chugai Pharmaceuticals Co. Ltd; Janssen Pharmaceuticals KK; Santen Pharmaceuticals Co. LTd; Pfizer Japan Inc: Astellas Pharma Inc; Daiichi-Sankyo Co,
8;
R. Fleischmann,
Pfizer Inc,
2,
Pfizer Inc,
8;
E. Keystone,
Abbott Laboratories; Amgen Inc.; AstraZeneca Pharmaceuticals LP; ,
2,
Abbott Laboratories; AstraZeneca Pharma, Biotest, Bristol-Myers Squibb Company; Centocor, Inc; F. Hoffmann-La Roche Inc; Genentech Inc; Merck, Nycomed, Pfizer Pharmaceuticals, UCB; ,
5;
J. M. Kremer,
Pfizer Inc,
2,
Pfizer Inc,
5;
C. Zerbini,
Novartis; Pfizer Inc.; Bristol-Myers Squibb; Eli-Lilly; Amgen; MSD,
2,
Pfizer Inc.; Bristol-Myers Squibb; Eli-Lilly; MSD,
5,
Pfizer Inc., Bristol-Myers Squibb,
6;
M. H. Cardiel,
Pfizer Inc,
2,
Pfizer Inc,
8,
Bristol Myers Squibb; Roche; Amgen; La Jolla Pharmaceutical,
9;
S. B. Cohen,
Genentech; Biogen-IDC; Merck; Sanofi-Aventis; Proctor Gamble; Pfizer; Centocor; Amgen; Scios; Bristol Myers Squibb; Wyeth Ayerst,
5,
Genentech; Biogen-IDC; Merck; Sanofi-Aventis; Proctor Gamble; Pfizer; Centocor; Amgen; Scios; Bristol Myers Squibb; Wyeth Ayerst,
9;
P. T. Nash,
Pfizer Inc,
2,
Pfizer Inc,
5,
Pfizer Inc,
8;
Y. Song,
None;
D. Tegzova,
None;
B. Wyman,
Pfizer Inc,
1,
Pfizer Inc,
3;
D. Gruben,
Pfizer Inc,
1,
Pfizer Inc,
3;
B. Benda,
Pfizer Inc,
1,
Pfizer Inc,
3;
G. Wallenstein,
Pfizer Inc,
1,
Pfizer Inc,
3;
S. Krishnaswami,
Pfizer Inc,
1,
Pfizer Inc,
3;
S. H. Zwillich,
Pfizer Inc,
1,
Pfizer Inc,
3;
J. Bradley,
Pfizer Inc,
1,
Pfizer Inc,
3;
C. A. Connell,
Pfizer Inc,
1,
Pfizer Inc,
3;
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tofacitinib-an-oral-janus-kinase-inhibitor-in-combination-with-methotrexate-reduced-the-progression-of-structural-damage-in-patients-with-rheumatoid-arthritis-year-2-efficacy-and-safety-results-fro/