Session Information
Date: Tuesday, October 23, 2018
Title: Vasculitis Poster III: Immunosuppressive Therapy in Giant Cell Arteritis and Polymyalgia Rheumatica
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: To evaluate the efficacy and safety of tocilizumab (TCZ) monotherapy for Large Vessel Vasculitis (LVV), including Takayasu arteritis (TAK) and Giant cell arteritis (GCA).
Methods: Twelve LVV patients (4 TAK patients and 8 GCA patients) who had been newly diagnosed at our hospital from January 2013 to May 2016 were enrolled in a prospective, open-label study. TCZ (8mg/kg) was administered intravenously every 2 weeks for the first 2 months and every 4 weeks for the next 10 months(total 15 times) without any corticosteroid(CS) or immunosuppressants(IS). Patients were followed for another 1 year after last TCZ administration without any treatment. The efficacy was assessed by clinical symptoms (fever, headache, fatigue, etc. ) and CRP level. Complete and partial response (CR and PR)was defined as disappearance or improvement of all clinical symptoms due to vasculitis and normalization of CRP(<0.3mg/dl). Poor clinical response was defined as patients who did not satisfy the criteria of CR/PR. Relapse of the disease was defined as the worsening or recurrence of clinical symptoms, increase of CRP attributable to activity of vasculitis, or initiation of CS and/or IS.
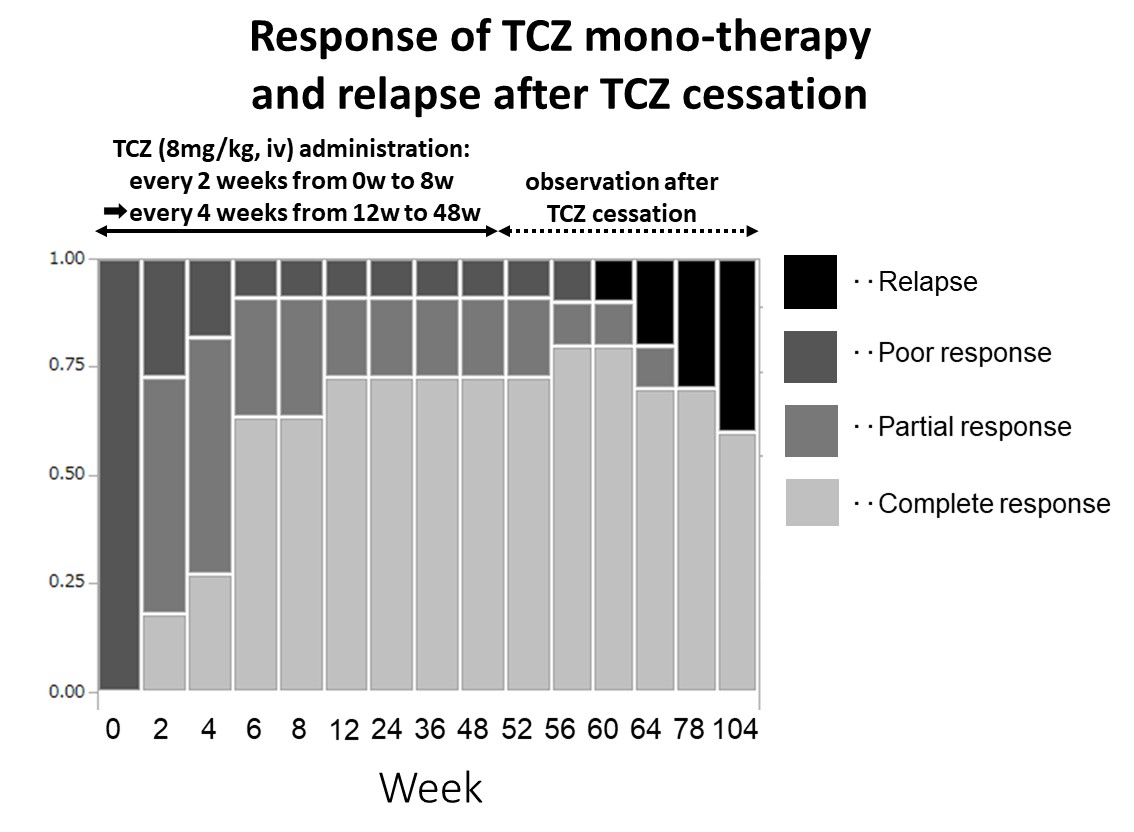
Results: The mean age was 58.9 y/o, the mean disease duration was 3.4 months, mean CRP was 6.0 mg/dl at the diagnose of the LVV. Efficacy of the TCZ monotherapy in active LVV was assessed in 11 patients, since 1 TAK patient chose not to participate in the study at week 2. Although 1 GCA patient had TCZ withdrawal due to heart failure at week 24, there were no other serious adverse events. LOCF (Last Observation Carried Forward) method was utilized to fill the missing data. Relapse after TCZ cessation was assessed in 10 patients who completed week 52. CR and PR rate were 73%/18% at week 24, and were maintained at week 52. Relapse occurred in 4 out of 10 patients (40%), although 60% completed week 104 after TCZ cessation. Response and relapses at each visit are summarized in figure 1.
Conclusion: This is the first study to show the effectiveness of merely IL-6 signal blockade in LVV patients, which could help us to understand the crucial role of IL-6 in the pathogenesis of LVV. TCZ monotherapy showed high response rate as an induction therapy for newly diagnosed LVV patients, and some of the patients did not require maintenance therapy after TCZ cessation. TCZ monotherapy might be an acceptable strategy as induction therapy for active LVV patients.
<Figure 1>
To cite this abstract in AMA style:
Saito S, Okuyama A, Okada Y, Shibata A, Sakai R, Chino K, Kurasawa T, Kondo T, Takei H, Amano K. Tocilizumab Monotherapy for Large Vessel Vasculitis: Results of 104-Week Treatment of a Prospective, Single-Center, Open Study [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/tocilizumab-monotherapy-for-large-vessel-vasculitis-results-of-104-week-treatment-of-a-prospective-single-center-open-study/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-monotherapy-for-large-vessel-vasculitis-results-of-104-week-treatment-of-a-prospective-single-center-open-study/