Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Malignancy is a potential risk of immunomodulatory treatments and may be increased in patients (pts) with rheumatoid arthritis (RA). The risk of malignancy was analyzed over long-term treatment with tocilizumab (TCZ) in clinical trials in adults with RA.
Methods: Assessment of malignancies was performed in all pts who received ≥1 dose of intravenous TCZ in 1 of 5 phase 3 placebo-controlled studies (OPTION, TOWARD, RADIATE, AMBITION, LITHE), a clinical pharmacology study, or long-term extension studies. In addition, 6-month data were included from the phase 4 TCZ monotherapy study (ADACTA). Data were pooled and analyzed from initial TCZ exposure to May 2, 2012 (cutoff). The indirect method of standardization was used to calculate age- and sex-adjusted standardized incidence ratio (SIR=ratio of observed to expected malignancies). The Surveillance Epidemiology and End Results (SEER) program of the US National Cancer Institute was used to calculate expected cases based on malignancy rates in a general population.
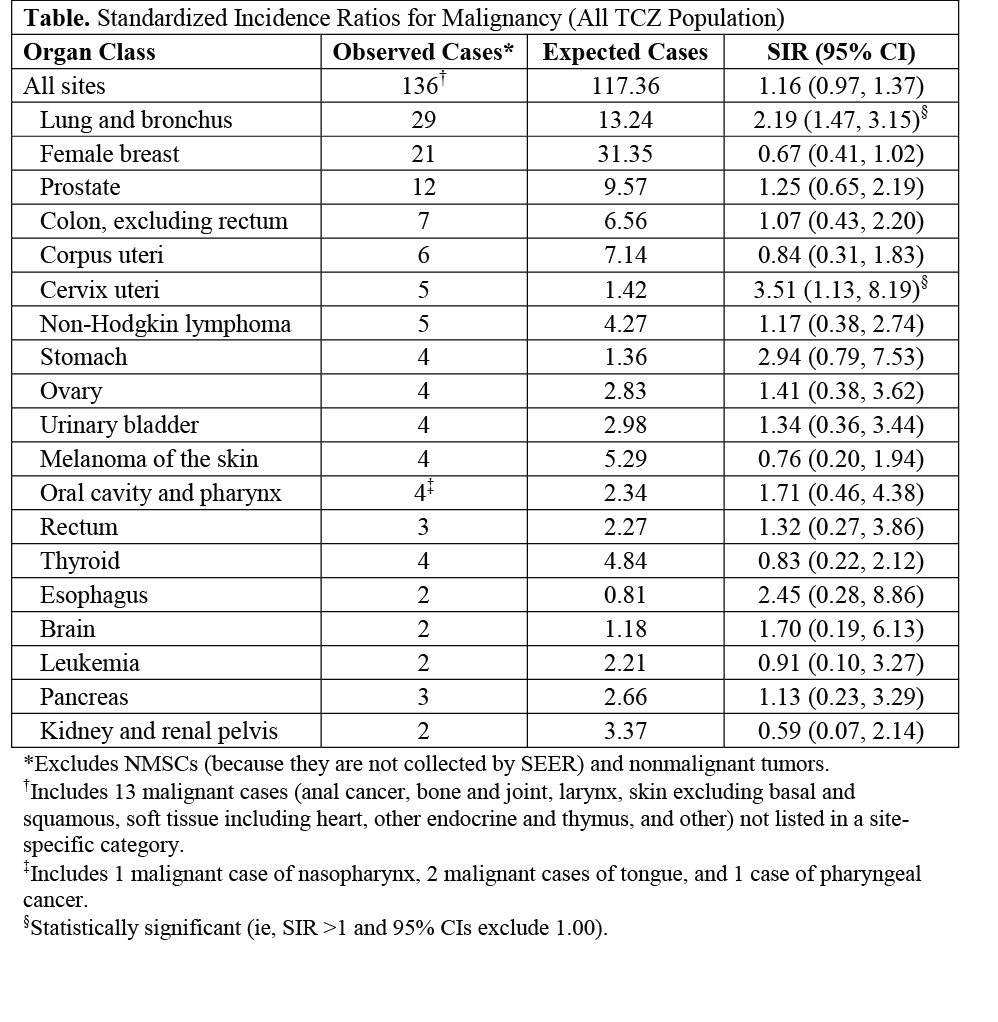
Results: In total, 4171 pts were included. Mean (median [range]) duration was 3.9 y (5.1 [0.0-6.8]); total observation time was 16,204.8 pt-y (PY). In addition to routine study monitoring for potential malignancy, investigators were queried via targeted questionnaire to ascertain details such as tissue diagnosis and biopsy results. Events with confirmed malignancy and those in which malignancy was not confirmed but could not be excluded were considered malignant for this analysis. The malignancy rate described above, including nonmelanoma skin cancer (NMSC), was 1.26/100 PY (95% CI: 1.09, 1.44) and remained stable over time. SIR for all malignancies combined (excluding NMSCs, which are not collected by SEER) and for individual tumor types showed that, with the exception of lung and cervical cancer, the observed number of malignancies was not statistically different (95% CI for SIR include 1.00)1 from that expected in the general population (Table). Meta-analysis of the incidence of malignancy in RA pts suggests that RA pts have a 63% increase in lung malignancy risk versus the general population.2 The observed cases of cervical cancer occurred in South America and Eastern Europe, regions with disproportionately high burdens of cervical cancer and underserved by national cervical cancer screening programs.3 As in other RA studies, the most frequent malignancy was NMSC: 42 basal cell carcinomas and 26 squamous cell carcinomas.
Conclusion: The rate of all malignancies in RA pts receiving long-term TCZ treatment has remained stable over time. To date, there is no evidence of higher than expected malignancy risk in RA pts treated with TCZ versus the general population.
References: 1. Jensen OM et al. Cancer Registration: Principles and Methods, 1991. 2. Smitten A et al. Arthritis Res Ther. 2008;10:R45. 3. Jemal A et al. CA Cancer J Clin. 2011;61:69-90.
Disclosure:
R. F. van Vollenhoven,
Abbott Immunology Pharmaceuticals,
2,
BMS,
2,
GSK,
2,
MSD,
2,
Pfizer Inc,
2,
Roche Pharmaceuticals,
2,
UCB,
2,
Abbott Immunology Pharmaceuticals,
5,
BMS,
5,
GSK,
5,
MSD,
5,
Pfizer Inc,
5,
Roche Pharmaceuticals,
5,
UCB,
5;
A. Rubbert-Roth,
Roche, UCB, MSD,
8,
Roche, Chugai, Pfizer,
2,
Roche, Chugai, Pfizer, UCB, MSD,
5;
A. Sebba,
Genentech, Lily, Novartis, Merck,
5,
Genentech, Novartis,
8;
B. Porter-Brown,
Roche Pharmaceuticals,
1,
Roche Pharmaceuticals,
3;
L. Rowell,
Roche Pharmaceuticals,
1,
Roche Pharmaceuticals,
3;
P. Napalkov,
Roche Pharmaceuticals,
1,
Roche Pharmaceuticals,
3;
D. Smart,
Roche Pharmaceuticals,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-in-patients-with-rheumatoid-arthritis-and-rates-of-malignancy-results-from-long-term-extension-clinical-trials/