Session Information
Date: Tuesday, November 7, 2017
Title: Rheumatoid Arthritis – Human Etiology and Pathogenesis Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In rheumatoid arthritis (RA) reduced muscle function clearly contributes to disability. Due to a lack of accurate in vitro RA models, the underlying mechanisms are not well understood. Moreover, current animal and cell culture systems are imperfect models of human disease, including RA, and do not recapitulate biologic and physiologic features of human skeletal muscle. Using engineered electrically responsive, contractile human skeletal muscle constructs (myobundles), we have established an in vitro 3D disease model of RA muscle to test the hypothesis that (1) myobundles derived from cells of RA patients show reduced capacity for repair and differentiation and reduced force production and (2) the overall decrement in function is due to select myokine (e.g. myostatin) and cytokine production.
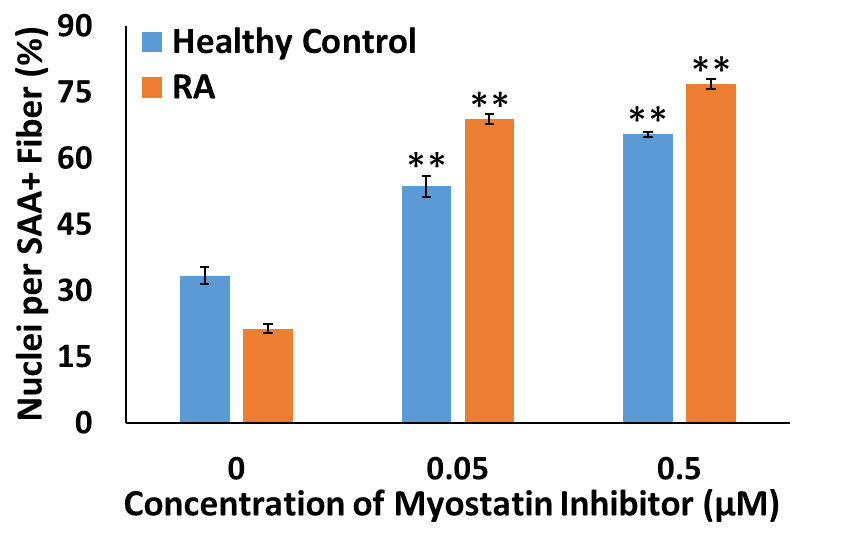
Methods: Myobundles were fabricated using vastus lateralis skeletal muscle cells from RA patients and healthy controls. To block myostatin and subsequent downstream signaling events, LDN-193189 was added at the transition from myoblasts to myotubes, after myobundle fabrication. After a 1- to 2-week differentiation period, tissue function was assessed by measuring myobundle twitch and tetanic contraction using a custom force measurement system. Muscle maturation was assessed by immunostaining myobundles for maturation markers, myosin heavy chain (MHC) or sarcomeric α-actinin (SAA). 2D studies were also performed with RA cells and healthy controls to quantify myoblast purity and percent nuclei per SAA-positive fiber. Unpaired t-tests were used to compare differences.
Results: Compared to healthy controls (n=4 donors) at 2-weeks differentiation, myobundles generated from RA vastus lateralis cells (n=3 donors) exhibited reduced twitch and tetanus forces and myosin heavy chain staining. Treatment with 0.05 µM LDN-19318979 in 2D culture improved maturation (RAD=47.5±1.5% % nuclei/SAA+ fiber versus ControlD=20.3±2.0%; Figure 1). Ten-day treatment of myobundles with 0.05 µM LDN-19318979 increased force production in RA myobundles (n=3 donors), but not in controls (n=3 donors).
Conclusion: Compared to healthy controls, RA myobundles exhibit reduced force production and MHC or SAA staining, indicative of reduced maturity and fiber formation. Treatment with LDN-19318979 increased RA myoblast fusion and myofiber formation in 2D and force production in 3D. RA muscle dysfunction may result from myostatin-mediated alterations in muscle remodeling.
To cite this abstract in AMA style:
Oliver CE, Davis BN, Hong J, Huffman KM, Truskey GA. Tissue-Engineered Human Skeletal Muscle Model of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/tissue-engineered-human-skeletal-muscle-model-of-rheumatoid-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tissue-engineered-human-skeletal-muscle-model-of-rheumatoid-arthritis/