Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: The first biosimilar etanercept (ETA-B) was approved in Canada in 2016, but real-world data comparing the effectiveness of ETA-B versus its equivalent originator (ETA-O) remain scarce. We compared time to remission and the prevalence of sustained remission in the first 12 of follow-up in rheumatoid arthritis (RA) cohorts.
Methods: We studied etanercept-naïve RA patients starting ETA-B or ETA-O between January 2015 and May 2022 from three prospective cohorts in Canada: the Rheumatoid Arthritis Pharmacovigilance Program and Outcomes Research in Therapeutics (RAPPORT), the Early Undifferentiated Polyarthritis (EUPA) cohort, and the RHUMADATA® registry. We restricted the analyses to patients with at least one follow-up visit after treatment initiation and with enough data to calculate remission. Remission was defined as Disease Activity Score 28 (DAS28) -CRP or -ESR ≤2.6, Simplified Disease Activity Index (SDAI) ≤ 3.3, or Clinical Disease Activity Index (CDAI) ≤ 2.8. We used Cox regression analysis to compare ETA-B versus ETA-O regarding time to first remission during follow-up (among patients without remission at baseline) and logistic regression to assess the prevalence of sustained remission (remission in two consecutive visits) in the first 12 months of follow-up. Models were adjusted for sex, age, body mass index (BMI), disease duration, and smoking status at cohort entry. Models were also adjusted for high/moderate disease activity, corticosteroid use, methotrexate (MTX), or hydroxychloroquine (HCQ), all at etanercept initiation.
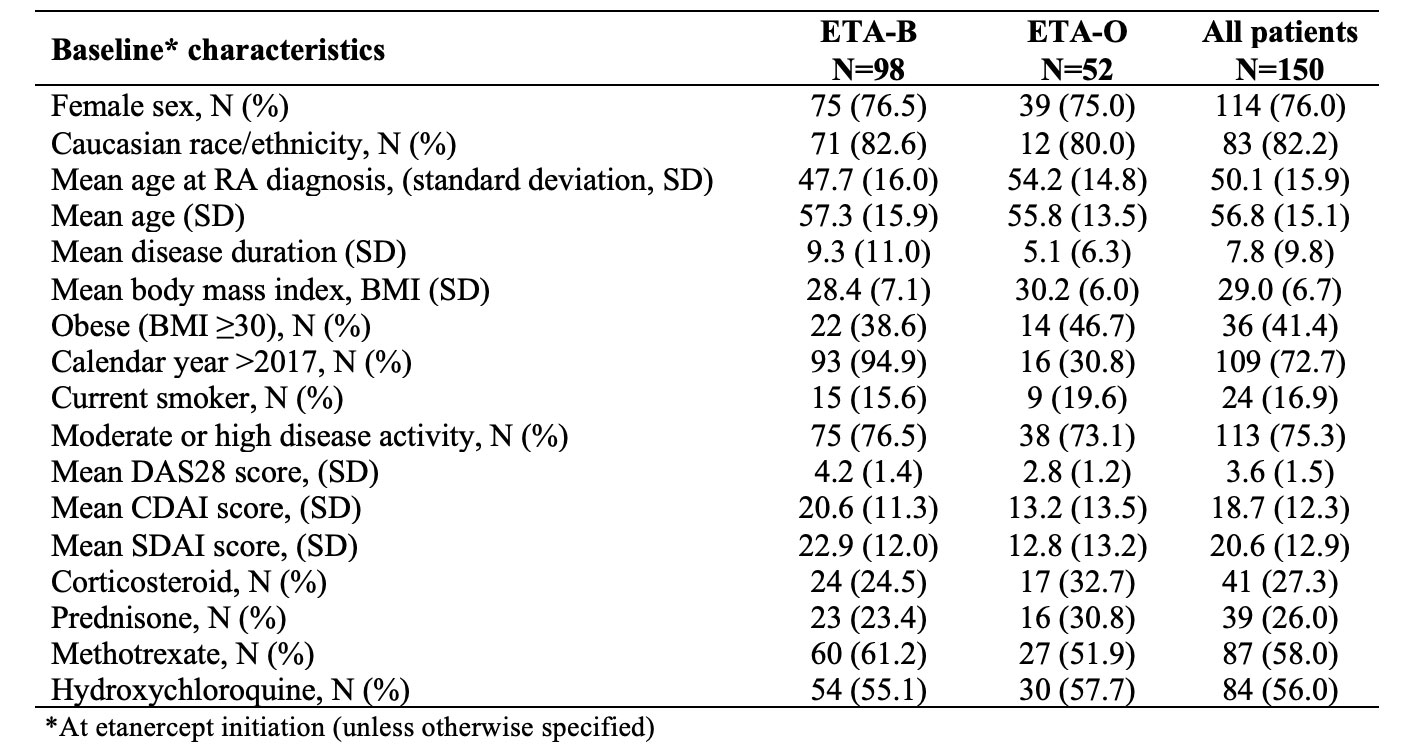
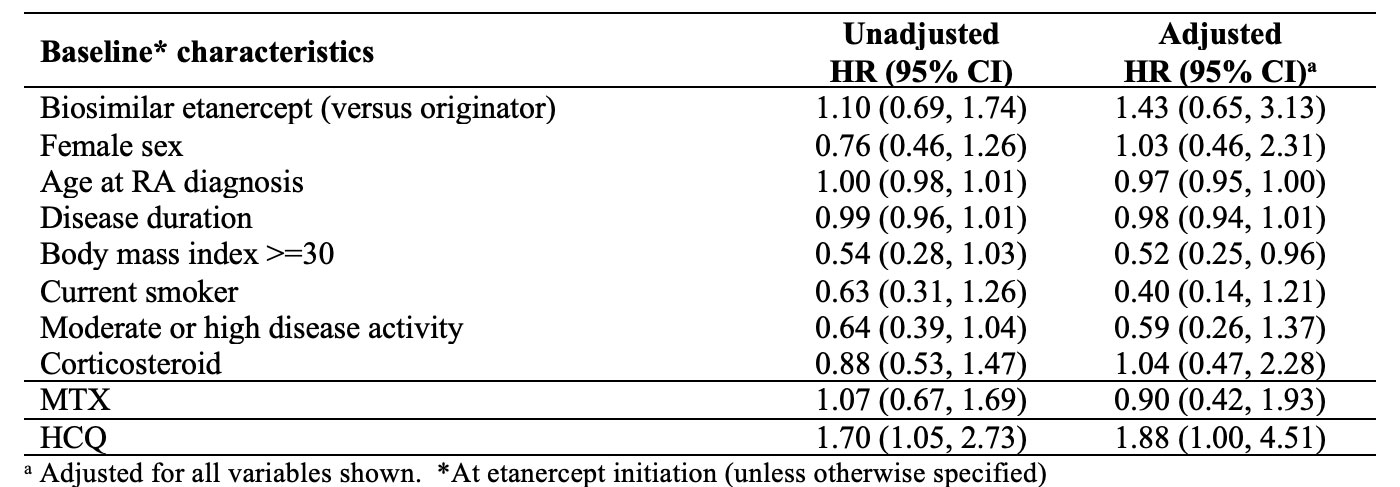
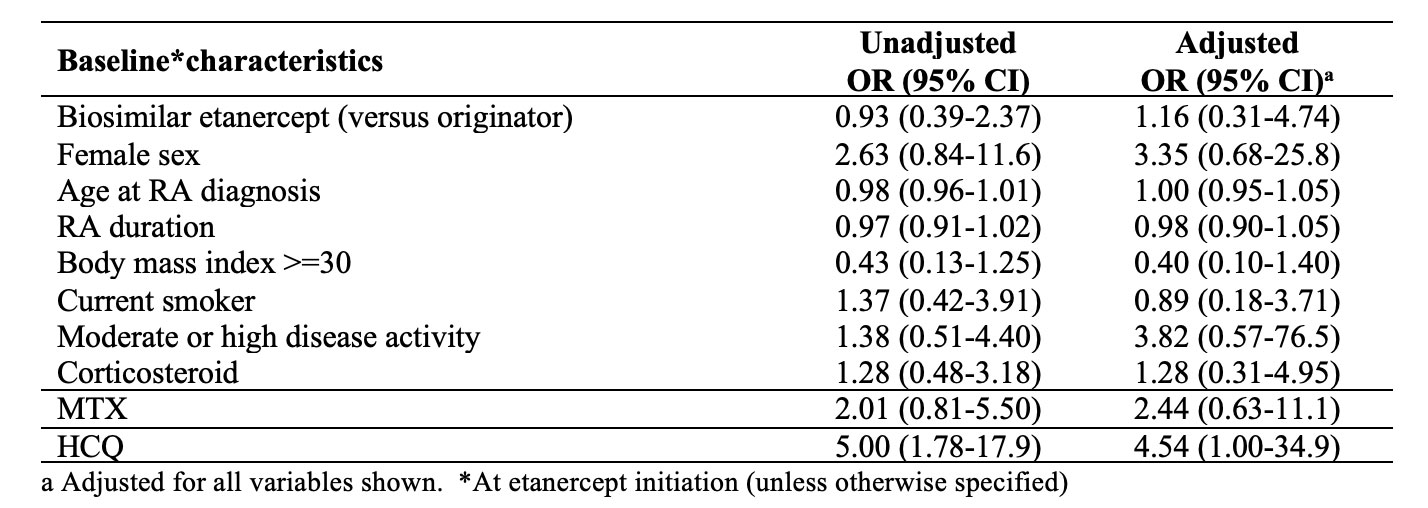
Results: We studied 150 RA patients initiating etanercept (65% on the biosimilar) between 2015-2022. Sex distribution was similar among ETA-B and ETA-O, but the biosimilar group has a longer disease duration and moderate/higher disease activity at baseline (Table 1). Among 125 patients without remission at baseline, the median time to first remission was 8.7 months with ETA-B versus 14.5 with ETA-O. In the multivariate analysis (Table 2), we were unable to detect a clear difference in achieving first remission when comparing ETA-B to ETA-O (hazard ratio, HR = 1.43, 95% confidence interval, CI 0.65-3.13). BMI >=30 was negatively associated with the outcome. Sustained remission in the first 12 months of follow-up was observed in 16.3% of ETA-B initiators versus 17.3% with ETA-O. After multivariate analysis (Table 3), there was no significant difference in the outcome between ETA-B and ETA-O (OR 1.16; 95%CI 0.31-4.74). The use of HCQ had a positive association with the outcome.
Conclusion: In this pooled analysis of three Canadian RA cohorts, we were unable to detect clear differences in achievement or sustained remission when comparing ETA-B and ETA-O.
To cite this abstract in AMA style:
Moura C, Lukusa L, Yan L, Maksymowych W, Choquette D, Boire G, Bernatsky S. Time to First Remission and Prevalence of Sustained Remission After Etanercept Biosimilar (ETA-B) or Originator (ETA-O) Initiation in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/time-to-first-remission-and-prevalence-of-sustained-remission-after-etanercept-biosimilar-eta-b-or-originator-eta-o-initiation-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/time-to-first-remission-and-prevalence-of-sustained-remission-after-etanercept-biosimilar-eta-b-or-originator-eta-o-initiation-in-rheumatoid-arthritis/