Session Information
Date: Tuesday, November 14, 2023
Title: (1945–1972) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Intravenous immune globulin (IVIG) recently received regulatory approval for the treatment of dermatomyositis, one of the idiopathic inflammatory myopathies (IIM). The pivotal randomized trial informing approval observed 6 thrombotic adverse events among patients exposed to IVIG, which included both venous thromboembolism (VTE) and arterial thromboembolism (ATE). The objective of this study is to determine the real-world incidence of ATE and VTE among adults who received IVIG for IIM or other indications.
Methods: Patients from TriNetX, a large federated health records database, were included if they received ≥1 IVIG administrations. ATE and VTE were identified using ICD-9-CM/ICD-10-CM codes and patients were characterized as IIM if they received two codes separated by 30 days within 3 years of the first IVIG administration. Patients could contribute multiple IVIg exposure intervals, which could be 1 to 7 days long, depending on how many consecutive IVIg administrations a patient received. Risk periods were defined for each patient for each IVIG interval as follows: ATE (IVIg interval day 1 to 2 days after the last day of the IVIG interval), VTE (IVIg interval day 1 to 13 days after the last day of the IVIg interval), and control (14 days after the last day of the IVIg interval to 31 days after IVIg interval day 1). Unadjusted incident rate ratios (IRR) were calculated and adjusted analyses were performed using stratified and weighted Poisson regressions.
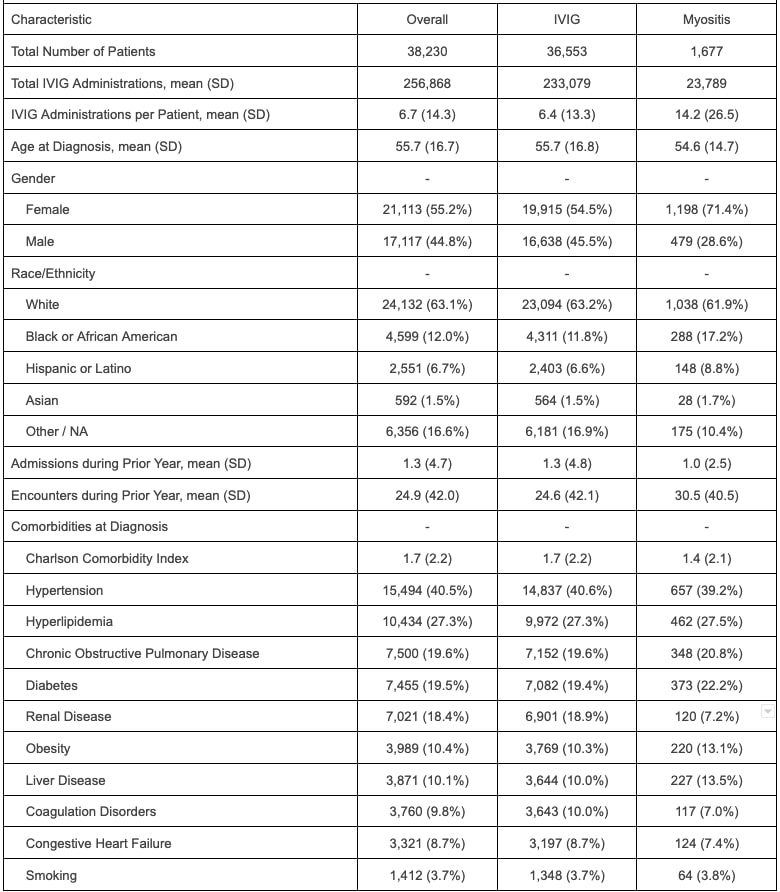
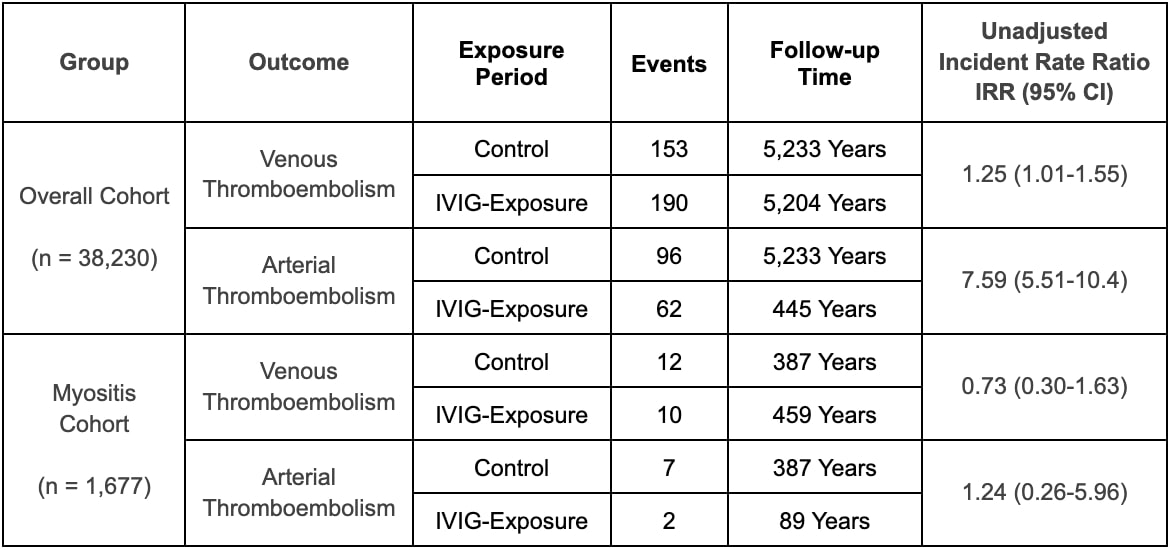
Results: This project included 38,230 patients who received a total of 256,868 IVIg administrations. A subset of 1,677 patients (4.4%) were categorized as IIM. The majority of IIM patients were female (71.4%) and white (61.9%). After excluding exposure time outside of the defined risk windows and censoring patients after their first event, there were 345 VTE and 158 ATE in the overall cohort. In the overall cohort, during the exposure and control periods the unadjusted incidence rates of VTE were 36.5 cases and 29.6 cases per 1,000 person-years, respectively (unadjusted IRR 1.23, 95% confidence interval (CI) 1.00-1.52) and the unadjusted incident rates of ATE were 139.0 cases and 18.3 cases per 1,000 person-years (unadjusted IRR 7.59, CI 5.51-10.40). Adjusted RR were 2.30 (95% CI 0.96-5.90) for VTE and 0.80 (95% CI 0.33-2.14) for ATE. Among patients with IIM, there were 22 VTE and 9 ATE. During exposure and control periods the unadjusted incidence rates of VTE were 21.8 cases and 31.0 cases per 1,000 person-years, respectively (unadjusted IRR 0.70, 95% CI 0.30-1.63) and the unadjusted incident rates of ATE were 22.4 cases and 18.1 cases per 1,000 person-years, respectively (IRR 1.24, CI 0.26-5.96). Adjusted rates could not be calculated for the subset with myositis given a low number of events.
Conclusion: In this study, the risk of ATE and VTE among patients exposed to IVIG was low. In unadjusted analysis, the rate of ATE and VTE was elevated after IVIG administration, but this did not persist in adjusted analyses. No increased risk was observed among patients with IIM in unadjusted analysis. Ongoing surveillance studies using randomized designs should be conducted to corroborate these findings.
To cite this abstract in AMA style:
Mehta R, Putman M, Saygin D. Thromboembolic Risk Associated with Intravenous Immune Globulin Use in Patients with Idiopathic Inflammatory Myopathy: A Large Database Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/thromboembolic-risk-associated-with-intravenous-immune-globulin-use-in-patients-with-idiopathic-inflammatory-myopathy-a-large-database-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/thromboembolic-risk-associated-with-intravenous-immune-globulin-use-in-patients-with-idiopathic-inflammatory-myopathy-a-large-database-study/