Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The ongoing phase 4 Pediatric Rheumatology Collaborative Study Group (PRCSG)/Paediatric Rheumatology INternational Trials Organisation (PRINTO) registry was designed to assess the long-term (up to 10 years) safety and efficacy of abatacept in pediatric patients with all JIA subtypes in a real-world setting.1 The objective of this analysis was to assess the real-world effectiveness of intravenous and subcutaneous abatacept over 3 years in patients with JIA who initiated treatment with abatacept within 1 month of enrollment in the PRCSG/PRINTO registry.
Methods: Using a standardized protocol and data collection process, clinical sites enrolled patients with JIA receiving/starting abatacept. For the current analysis, all patients with JIA who initiated abatacept within 1 month of enrollment in the PRCSG/PRINTO registry were included. Effectiveness was assessed at baseline and at 3, 6, 9, 12, 24, and 36 months. Outcomes include physician global assessment of disease activity, overall well-being score, number of active joints and joints with limited range of motion, the clinical 10-joint Juvenile Arthritis Disease Activity Score (cJADAS10), and JIA-ACR30, 50, 70, and 90. The cJADAS10 used validated cut-offs for low disease activity (LDA; ≤ 3.8), inactive disease (ID; ≤ 1.0), and remission (ID for ≥ 6 months). An as-observed analysis is presented.
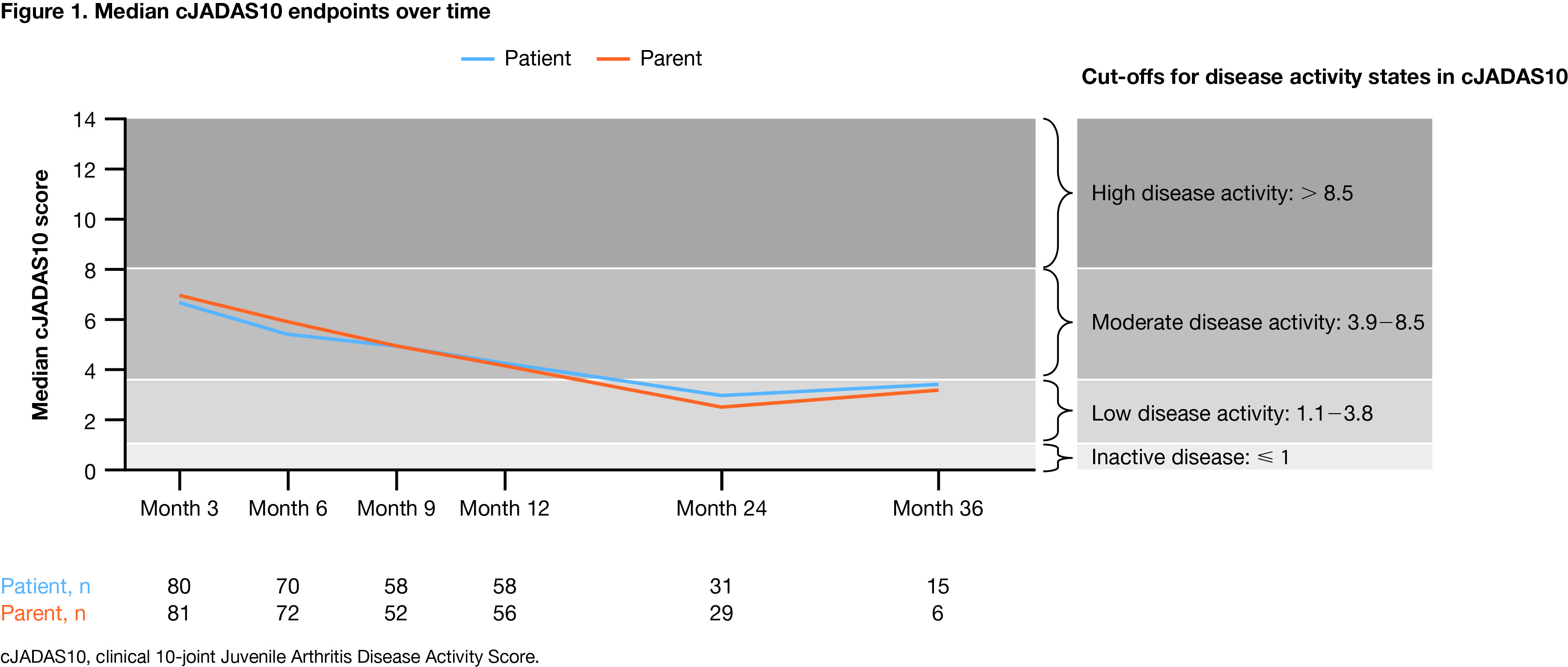
Results: As of March 31, 2020, 134 patients who initiated abatacept within 1 month of enrollment in the PRCSG/PRINTO registry were included in this analysis. Of these, 110 (82.1%) were female, the baseline mean (median) age at enrollment was 12.8 (13.1) years, the time since JIA diagnosis was 60.2 (45.6) months, and the time on abatacept treatment at baseline was 0.28 (0.03) months. Median cJADAS10 scores decreased from month 3 to month 36 and the proportions of patients achieving cJADAS10 LDA and cJADAS10 ID increased over time (Figure 1). JIA-ACR 30, 50, 70, and 90 responses were seen as early as month 3 and were sustained over time to month 24; the percentage of patients achieving these responses at month 36 was influenced by the relatively low number of patients (due to ongoing enrollment and loss to follow-up) available for the analysis (Table 1). In addition, physician global assessment of disease activity and number of active joints decreased over time (Table 1).
Conclusion: In patients with JIA who initiated abatacept within 1 month of joining the PRCSG/PRINTO registry, there were rapid, clinically relevant responses, such as improvement in cJADAS10 scores, number of active joints, number of joints with a limited range of motion and overall well-being. These improvements were sustained over 24 months.
Reference:
1. Lovell DJ, et al. ACR 2020. Abstract 0714.
Medical writing: Claire Line, PhD (Caudex), funded by Bristol Myers Squibb
To cite this abstract in AMA style:
Ruperto N, Brunner H, Tzaribachev N, Orbán I, Staņēviča V, Quintero del Rio A, Quartier P, Huber A, Kietz D, Dare J, Kingsbury D, Graham T, Foeldvari I, Patel J, Dominique A, Dong L, Kou T, Wong R, Martini A, Lovell D. Three-year Effectiveness in Patients with JIA Initiating Abatacept: Results from the PRCSG/PRINTO JIA Real-World Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/three-year-effectiveness-in-patients-with-jia-initiating-abatacept-results-from-the-prcsg-printo-jia-real-world-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/three-year-effectiveness-in-patients-with-jia-initiating-abatacept-results-from-the-prcsg-printo-jia-real-world-registry/