Session Information
Date: Tuesday, November 9, 2021
Title: Abstracts: Spondyloarthritis Including PsA – Treatment II: Biologic Therapies (1943–1946)
Session Type: Abstract Session
Session Time: 4:45PM-5:00PM
Background/Purpose: Proactive therapeutic drug monitoring (TDM), a treatment strategy based on scheduled assessments of serum drug levels, has been proposed to optimize efficacy and safety of infliximab and other TNF inhibitors. It is still unclear whether proactive TDM improves clinical outcomes. The NOR-DRUM trials (A and B) are the first randomized trials to assess the impact of proactive TDM of infliximab in a range of immune-mediated inflammatory diseases. The NOR-DRUM A trial focused on the induction phase of infliximab.1 The aim of the present trial, NOR-DRUM B, was to assess effectiveness of TDM during maintenance infliximab therapy.
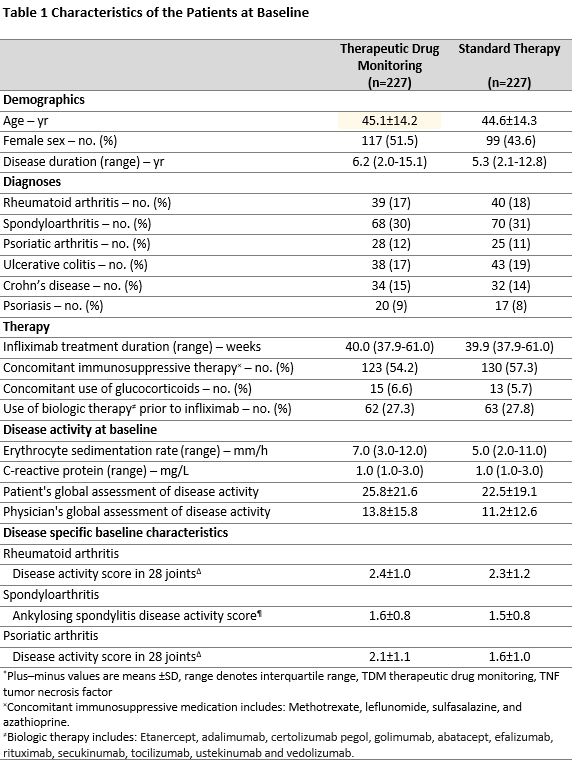
Methods: In this 52-week randomized, open-label, multicentre trial, adult patients with rheumatoid arthritis (n=79), spondyloarthritis (n=138), psoriatic arthritis (n=53), ulcerative colitis (n=81), Crohns disease (n=66) or psoriasis (n=37) on infliximab therapy for a minimum of 30 weeks were randomly assigned 1:1 to infliximab based on TDM or standard therapy. In the TDM group, infliximab doses- and intervals were adjusted according to an algorithm to maintain serum infliximab levels within the prespecified therapeutic range 3-8 mg/L. In the standard therapy group, administration of infliximab was based on clinical judgement. The primary endpoint was sustained disease control without disease worsening. Disease worsening was defined by disease specific composite scores or a consensus on disease worsening between investigator and patient leading to major change in treatment. The primary analysis was performed by mixed effect logistic regression.
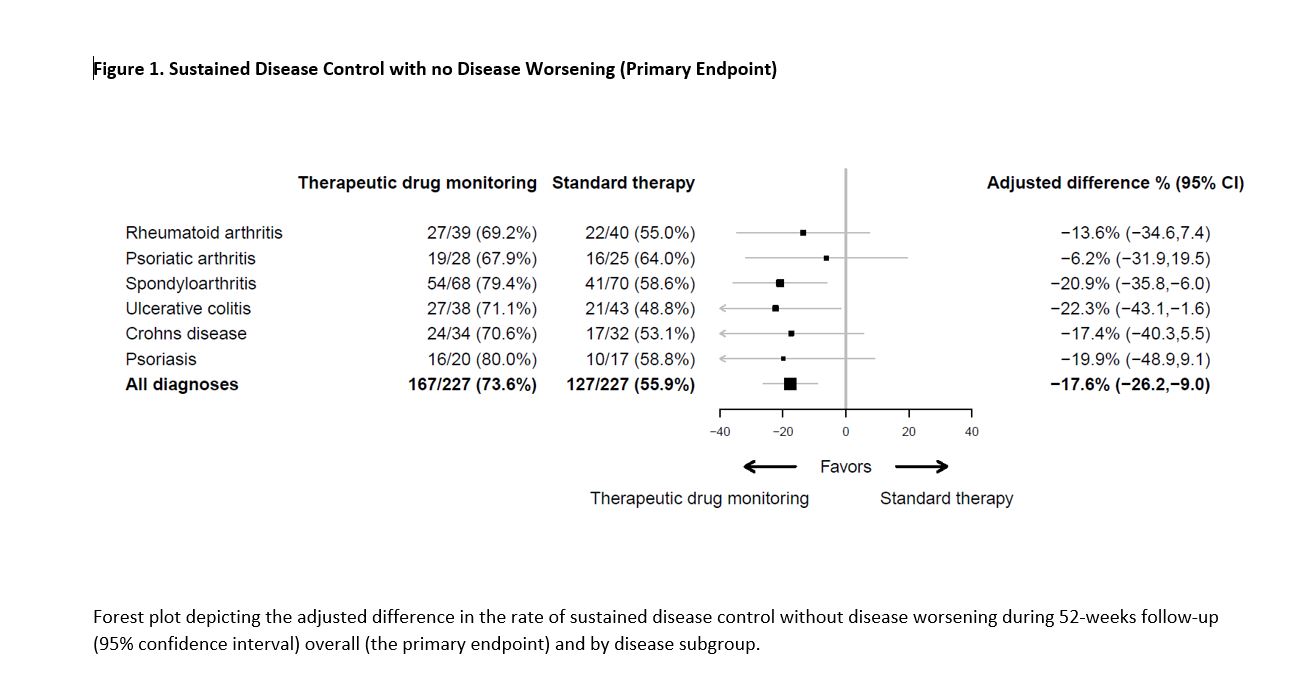
Results: Between June 7, 2017, and December 12, 2019, 458 patients were randomized and 454 received the allocated strategy and were included in the primary analyses. During the 52-week follow-up, the primary endpoint of sustained disease control without disease worsening was achieved in 167 (73.6 %) patients in the TDM group compared to 127 (55.9%) patients in the standard therapy group. The estimated adjusted difference was -17.6% (95% confidence interval -26.2 to -9.0, p=< 0.001). (Figure 1) Results were consistent in sensitivity analyses. The two groups were balanced regarding baseline characteristics (Table 1). Time to disease worsening was different in the two groups, hazard ratio (95% CI) 2.1 (1.5-2.9) (Figure 2). Secondary endpoints comparing disease activity and patient reported outcomes at week 52 did not show significant differences between the groups. Mean infliximab dose received during the trial was 4.8 mg/kg in both groups. Twenty-one (9.2%) in the TDM group and 27 (15.0 %) in the control group developed clinically significant levels of anti-drug antibodies. Adverse events were reported in 137 (60%) and 142 (63%) patients in the TDM and standard therapy groups, respectively.
Conclusion: Proactive TDM proved more effective than standard therapy in sustaining disease control without disease worsening. These results support implementation of proactive TDM as a general strategy during maintenance therapy with infliximab and have the potential to change clinical practice across specialities.
1Syversen SW et al. JAMA. 2021
To cite this abstract in AMA style:
Syversen S, Goll G, Jørgensen K, Brun M, Sandanger , Hammersbøen K, Sexton J, Olsen I, Gehin J, Warren D, Klaasen R, Bruun T, Ljoså M, Haugen A, Njålla R, Michelsen B, Zettel C, Bragenes Y, Skorpe S, Strand E, Mielnik P, Mørk C, Kvien T, Jahnsen J, Bolstad N, Haavardsholm E. Therapeutic Drug Monitoring Compared to Standard Infliximab Therapy in Patients with Immune-mediated Inflammatory Diseases: A Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/therapeutic-drug-monitoring-compared-to-standard-infliximab-therapy-in-patients-with-immune-mediated-inflammatory-diseases-a-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/therapeutic-drug-monitoring-compared-to-standard-infliximab-therapy-in-patients-with-immune-mediated-inflammatory-diseases-a-randomized-controlled-trial/