Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The advent of biologics such as IL-1 receptor antagonists has dramatically improved outcomes for children with pediatric rheumatic diseases and hyperinflammation. However, there remains a subset of patients with systemic juvenile idiopathic arthritis (sJIA) who develop life-threatening complications of macrophage activation syndrome (MAS) and interstitial lung disease (ILD) despite treatment with glucocorticoids and IL-1 inhibition. Recent findings point to robust interferon and IL-18 signature as drivers of persistent inflammation in sJIA, suggesting a potential role for inhibition of these pathways using Janus kinase inhibitors, such as ruxolitinib, as a viable therapeutic strategy.
Methods: We completed a retrospective chart review on three patients with sJIA or sJIA-like disease started on ruxolitinib for improved disease control or to allow for glucocorticoid weaning.
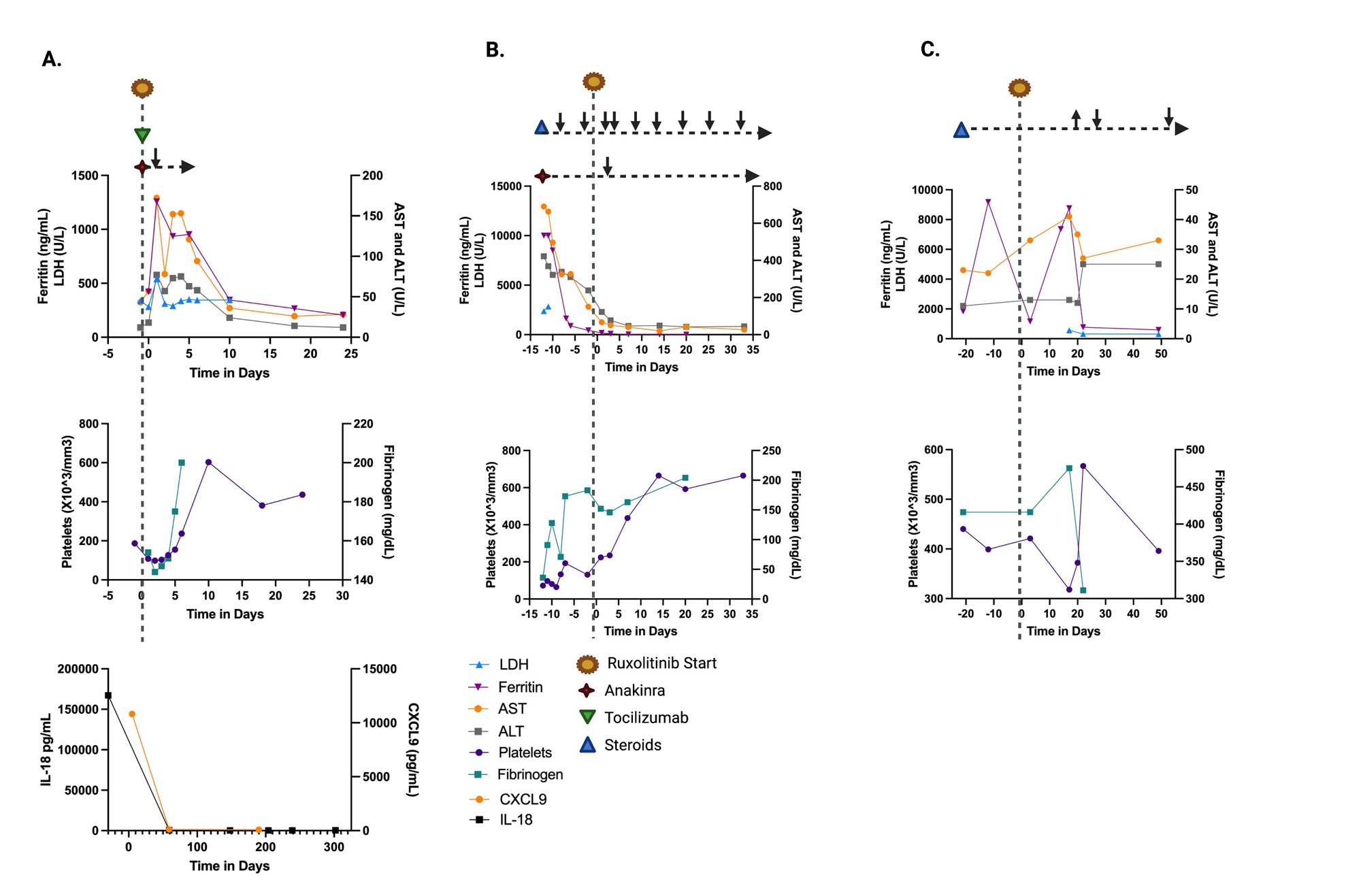
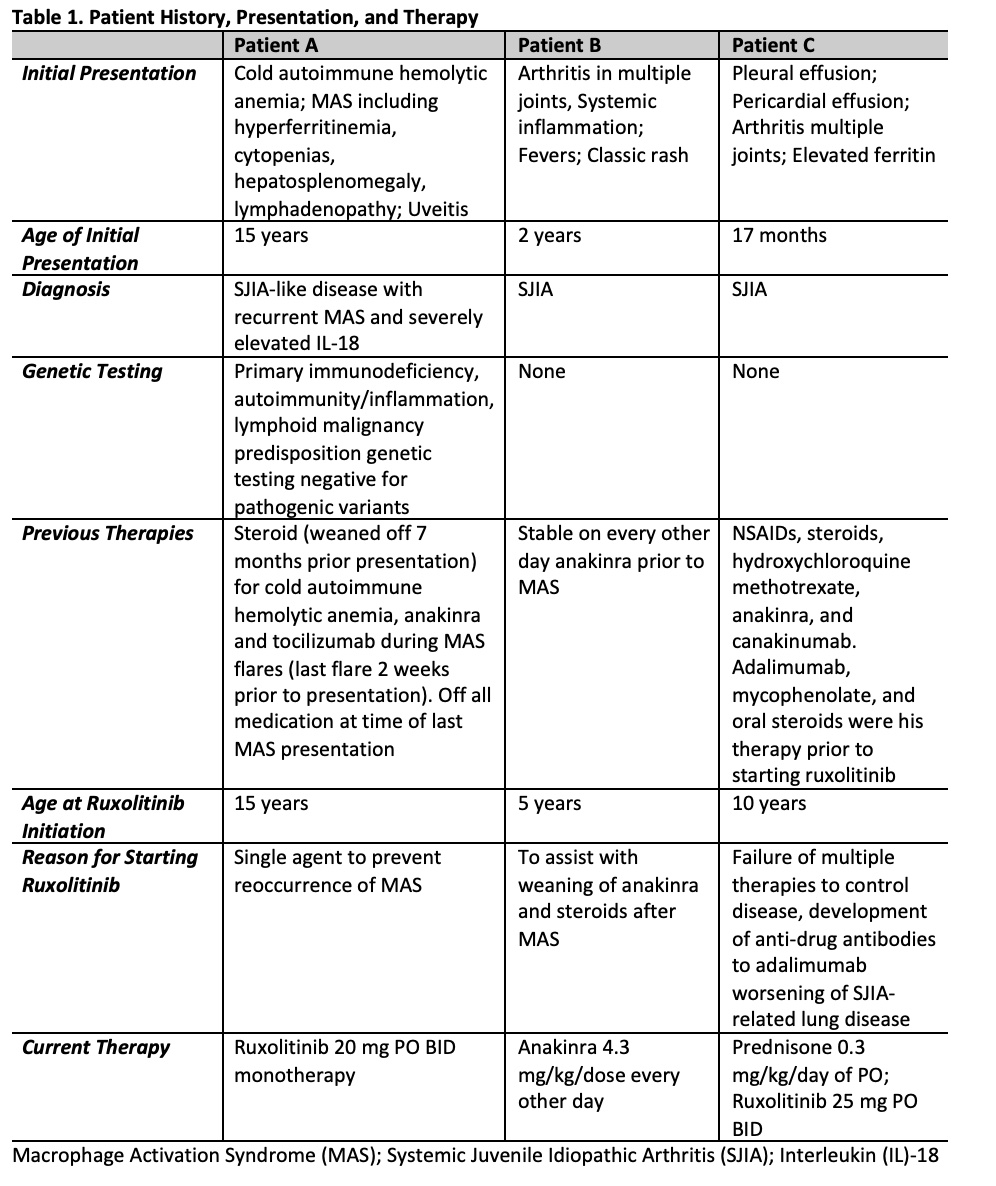
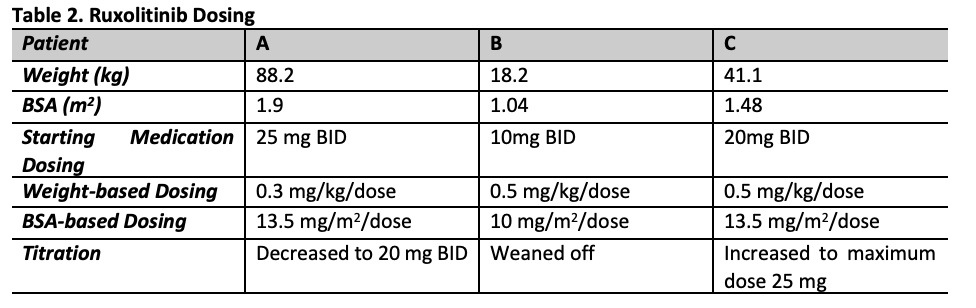
Results: Patients ages were 5-15 years of age with 2 females and 1 male (Table 1). Patient A with sJIA-like disease presented with recurrent MAS with fever and hypotension requiring vasopressor support and was treated with ruxolitinib, anakinra, and tocilizumab and MAS was controlled (Figure 1). Anakinra was weaned off prior to discharge home. Ruxolitinib was continued as a single agent and has maintained excellent control of the patient’s disease with no further MAS flares or adverse events for the last 10 months. Patient B with previously diagnosed sJIA presented with fulminant MAS secondary to EBV. She was treated with pulse dose steroids and anakinra with some initial stabilization. Ruxolitinib was started due to lack of further improvement in MAS. The addition of ruxolitinib controlled her MAS and steroid and anakinra doses were tapered (Table 2; Figure 1). Oral steroids were discontinued 9 weeks after starting ruxolitinib. Ruxolitinib was discontinued at 3 months. Patient was continued on anakinra. Patient C with previously diagnosed sJIA developed sJIA-associated lung disease on canakinumab therapy. Ruxolitinib was added to his therapy (Table 1, 2) due to continued systemic inflammation and inability to wean steroids. Within the 4 weeks the patient reported improvement in joint pain and had no oxygen desaturations with walking. Within 6 weeks, lung function improved with FEV1 increasing from 85% to 91% predicted. Ruxolitinib dose was increased to 25 mg twice a day at 4 months based on patient toleration and to optimize disease control, allowing successful weaning of steroids by 50%. Patients have not had any infectious complications while on ruxoltinib. One adverse event reported was Grade 1 elevated cholesterol. Mean follow-up time is 214 days (146-322). Mean time patients were on ruxolitinib was 189 days (100-322).
Conclusion: Ruxolitinib was well tolerated in these patients with sJIA or sJIA-like disease with no significant adverse events, including cytopenias, transaminitis, or infections. Use of ruxoltinib provided improved disease control as a single agent or in combination with other immunomodulators and allowed reduction of other therapies, such as steroids. Based on our experience, patients may require and can tolerate higher doses than previously used for other indications with titration up and down to affect.
To cite this abstract in AMA style:
Collins K, Abutineh I, Paul T, Rai P, Schulert G, Hines M. The Use of Ruxolitinib for Improved Disease Control in Systemic Juvenile Idiopathic Arthritis (sJIA) and Recurrent Macrophage Activation Syndrome (MAS) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-use-of-ruxolitinib-for-improved-disease-control-in-systemic-juvenile-idiopathic-arthritis-sjia-and-recurrent-macrophage-activation-syndrome-mas/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-use-of-ruxolitinib-for-improved-disease-control-in-systemic-juvenile-idiopathic-arthritis-sjia-and-recurrent-macrophage-activation-syndrome-mas/