Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient-reported outcome measures (PROMs) are important tools for evaluating disease activity, functional status, and quality of life in patients with psoriatic arthritis (PsA). The Psoriatic Arthritis Impact of Disease (PSAID) questionnaire is a commonly used PROM. The Psoriatic Arthritis Response Criteria (PSARC) is a composite measure that assesses changes in disease activity based joint pain, swelling and general health. The objective of this study was to assess the correlation between remote PSAID-12 and in-person tender, swollen joint and pain in patients with PsA attending the clinic.
Methods: This study was a cross-sectional, observational study of patients with PsA meeting CASPAR critieria. Patients were recruited consecutively from clinic and completed the PSAID-12 questionnaires 14 days prior to their clinic visit digitally or by paper. Demographic and clinical data, including age, gender, disease duration, medication were collected. The tender (68 joint) and swollen (66 joint) count as well as the Likert Pain Score was conducted in-person in the clinic. The primary outcome was the correlation between PSAID-12 and tender, swollen joint and Likert Pain Score. Descriptive statistics was used to summarise the demographic and clinical characteristics of the study population. Pearson correlation coefficients were calculated to assess the correlation between PSAID-12 and tender joint, swollen joint and pain scores.
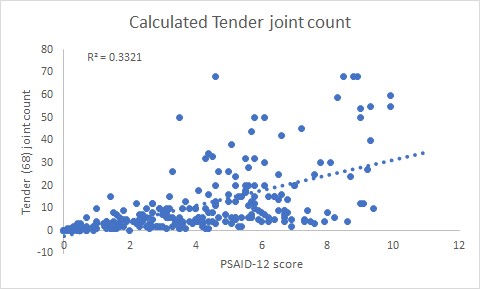
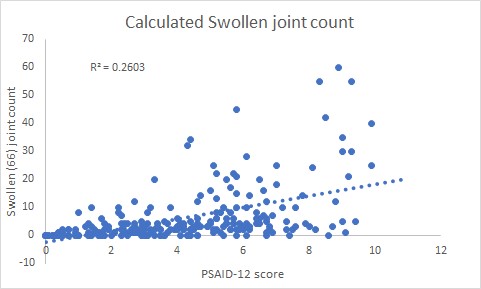
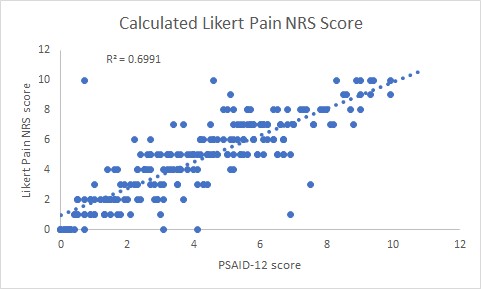
Results: The total number of patients in the study was 235. The female:male ratio was 1.0:0.8. The mean disease duration was 27 years (range 2 months to 54 years). The mean age was 52 years (range 18 to 86 years). The number of patients on cs DMARDs were 201/235 (85.5%) and bDMARDs 84/235 (35.7%). The mean PSAID-12 score was 4.1 (SD 2.5). The number of patients in remission (PSAID-12 < 1.4) was 48 (21%), low disease activity (PSAID-12, 1.4- 4) 71 (30%), moderate disease activity (PSAID-12, 4-6.7) was 83 (35%), active disease (PSAID-12 > 6.7) was 33 (14%), tender joint count 11.7 (SD 14.8), swollen joint count 6.1 (SD 10.1) and Likert Pain Score 4.6 (SD 2.6). There was a positive correlation between the pre-clinic PSAID-12 score and the in-person calculated tender joint count (r=0.58, p < 0.05, figure 1)swollen joint count (r= 0.51, p < 0.05, figure 2) and the Likert pain score (r=0.84, t-score 22.7, p< 0.05, figure 3).
Conclusion: This real-world clinic study confirms the findings from the previous PSAID-12 validation studies where the PSAID-12 correlation with Pain VAS was r = 0.83, tender joint count was r = 0.57 and swollen joint count was r = 0.40. Our study has shown very similar results. This provides evidence that the PSAID-12 score may be used as a PROM for remote monitoring during the longer intervals between in-person clinic reviews. Using PSAID-12, one-fifth of patients were in PsA remission and may be suitable for a remote or tele-consultation which will reduce the need to travel to hospital for an in-patient review. The results of this study may help to improve the understanding of the relationship between these remote monitoring and in-person assessments and their utility in clinical practice for assessing disease activity and functional status in patients with PsA.
To cite this abstract in AMA style:
Chan A, Nwe N. The Use of PSAID-12 in Remote Monitoring Correlates with In-person Clinical Examination Findings in Psoratic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-use-of-psaid-12-in-remote-monitoring-correlates-with-in-person-clinical-examination-findings-in-psoratic-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-use-of-psaid-12-in-remote-monitoring-correlates-with-in-person-clinical-examination-findings-in-psoratic-arthritis/