Session Information
Date: Saturday, November 12, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster I: Giant Cell Arteritis
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Giant cell arteritis (GCA) is the most common vasculitis in the age-group 50 years and older. Cranial involvement is common and can lead to devastating ischemic complications, such as vision loss and stroke. Glucocorticoids (GCs) are the cornerstone of GCA treatment. Today, patients with visual complications are treated with high dose intravenous (IV) methylprednisolone (MP). GC treatment is associated with several treatment related toxicities and comorbidities. We aim to study characteristics and outcome of patients with GCA receiving IV MP, in terms of treatment effectiveness, comorbidities and mortality.
Methods: In this retrospective 10-year study, patients with biopsy confirmed GCA from the southernmost region of Sweden receiving IV MP were compared to patients only treated with oral GCs. All patients diagnosed between 2010 and 2019 that had been hospitalized at one of the region’s 4 largest hospitals within 1 month prior to or 3 months after GCA diagnosis, were included. Data on characteristics at diagnosis, treatment and ophthalmologic examinations were obtained through case record reviews. Information on occurrence of comorbidities after GCA and mortality through December 31, 2020, were extracted from relevant healthcare registers.
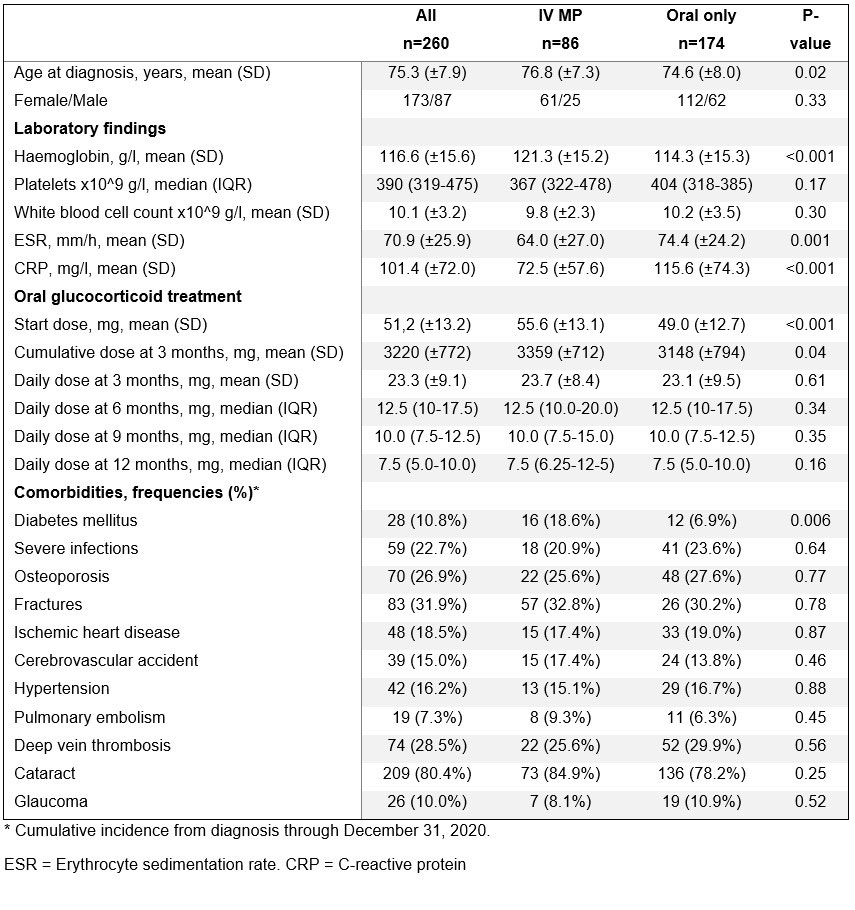
Results: Two-hundred-sixty patients were included, 86 treated with IV MP followed by oral GCs and 174 receiving oral GCs only. Patients treated with IV MP were on average older at diagnosis, and had a lower inflammatory response, measured by ESR and CRP (Table). Compared to the patients receiving oral GCs only, patients treated with IV MP received a higher mean starting dose of oral GCs, 55.6 mg vs. 49.0 mg (p< 0.001), but there was no such difference at later follow-up (Table). The IV MP group also had a higher mean cumulative oral GC dose at 3 months, 3359 (SD ±712) vs 3148 (SD ±794) mg (p=0.04). In total, 116 (45%) patients reported visual symptoms at onset. Eighty-three (97%) of patients treated with IV MP had visual symptoms compared to only 33 (19%) of those treated with only oral GC. Out of the patients with visual symptoms, 45 (89 eyes) went through both an initial ophthalmological exam and a 3-month follow-up, 37 (73 eyes) received IV MP treatment and 8 (16 eyes) were treated with oral GCs only. No significant improvement was seen in visual acuity from onset to follow-up, overall (0.48 at onset compared to 0.52 at follow-up; p=0.13), in the IV MP treated patients (0.46 vs 0.48; p=0.46) or the oral only group (0.56 vs 0.68; p=0.07). Patients treated with IV MP had a higher occurrence of diabetes mellitus later in life, but not of other comorbidities (Table). There was no difference between groups in survival (Figure).
Conclusion: Among patients with biopsy-proven GCA, those treated with IV MP were mainly patients with visual symptoms. In such patients, visual acuity did not change significantly over 3 months. Patients receiving IV MP had higher cumulative oral GC at 3 months and higher incidence of diabetes mellitus than those treated with oral GCs only. Optimal treatment to prevent visual loss and limit GC toxicity in GCA should be further investigated.
To cite this abstract in AMA style:
Henningson H, Hammar B, Turesson C, Mohammad A. The Use of Intravenous Methylprednisolone in Patients with Giant Cell Arteritis: A Population-Based Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-use-of-intravenous-methylprednisolone-in-patients-with-giant-cell-arteritis-a-population-based-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-use-of-intravenous-methylprednisolone-in-patients-with-giant-cell-arteritis-a-population-based-study/