Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Reporting of treatment-emergent adverse events (TEAEs) in RA clinical trials can be summarized as exposure-adjusted incidence rates (EAIRs) or exposure-adjusted event rates (EAERs). Censored EAIR (EAIR), weighing exposure up to a patient’s first event, is commonly reported; uncensored EAIR (EAIRu), using total exposure time for all patients, can also be used. For EAIR, exposure time can vary by event. In contrast to EAIR(u), the total number of events are used to calculate EAER. The 3 methods account for different exposures and/or multiple events, which can impact the outcome evaluation. Studies of filgotinib (FIL) in RA1 report safety data as EAIR/100 patient-years of exposure (PYE) for TEAEs, which is uncensored. The objective of this study was to describe the outcome of long-term FIL integrated safety data in RA by applying different statistical methodologies: EAER, EAIRu, and EAIR.
Methods: Integrated FIL safety data from 7 clinical trials were assessed.1 Predefined adverse events of special interest (AESIs) included serious infections (any), herpes zoster (HZ), major adverse cardiac events (MACE), malignancies (excluding nonmelanoma skin cancer [NMSC]), NMSC, and venous thromboembolism (VTE). The number of patients with an event, number of events, EAER, EAIRu, and EAIR were summarized. The data extraction date was January 2021 for the DARWIN 3 (NCT02065700) long-term extension (LTE) and November 2020 for the FINCH 4 (NCT03025308) LTE.
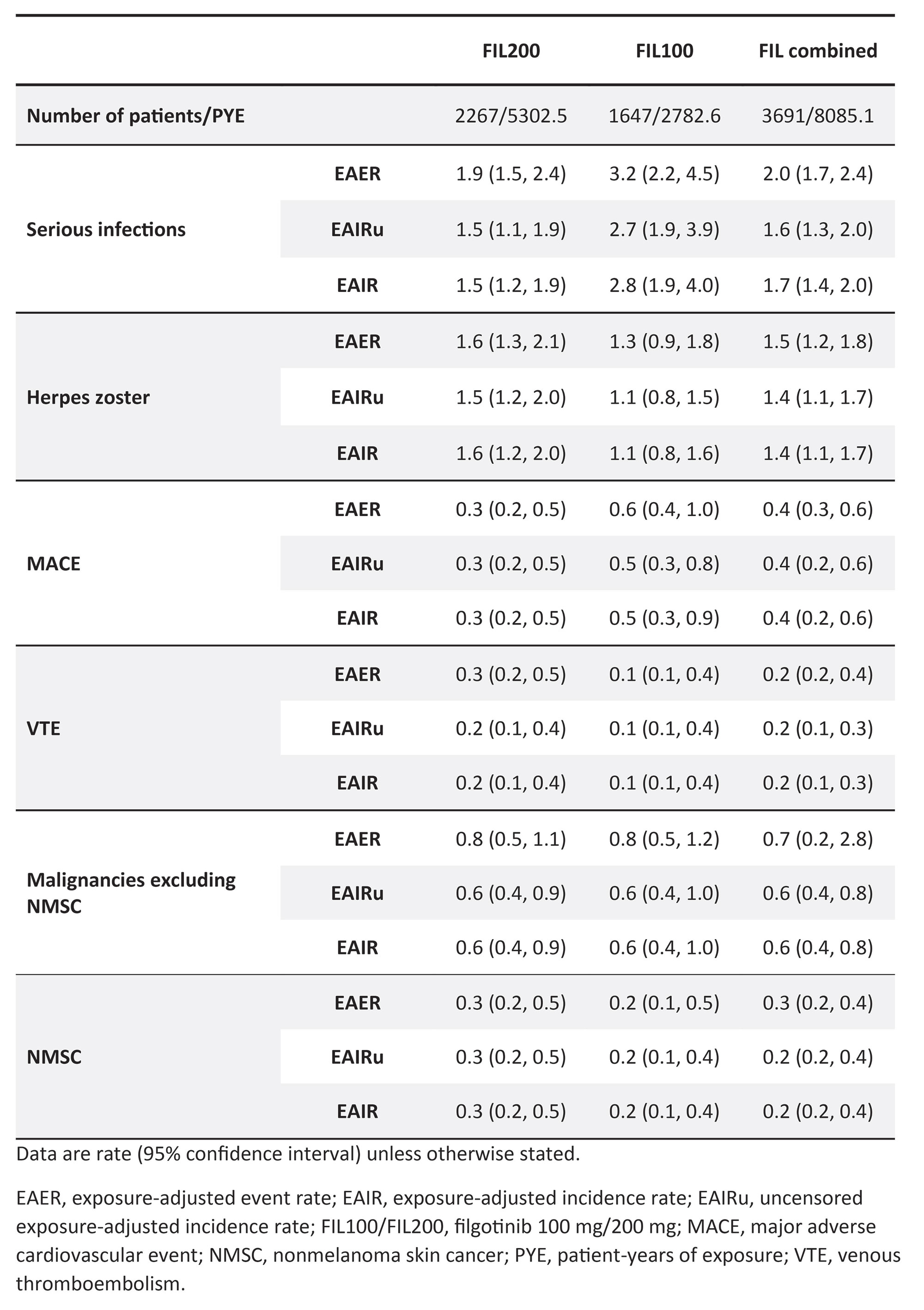
Results: In total, 3691 patients received ≥1 FIL dose for 8085 PYE. In this population, 176 serious infections were reported in 137 patients, 125 HZ events were reported in 112 patients, 39 MACE were reported in 33 patients, 20 cases of VTE were reported in 15 patients, 60 malignancies excluding NMSC were reported in 49 patients, and 21 cases of NMSC were reported in 20 patients. Within each treatment arm (FIL 200 mg [FIL200], FIL 100 mg [FIL100], or combined FIL), rates for most AESIs were similar when reported as EAER, EAIRu, or EAIR (Table). For serious infections, EAER was higher than EAIRu or EAIR. The total exposure time to first event (censored PYE) was high and comparable to total exposure (PYE) ( >2700 years and >5100 years for the total populations in the FIL100 and FIL200 groups, respectively).
Conclusion: These data confirm that using different methods to analyze FIL safety data (EAER, EAIRu, EAIR) does not result in different safety outcomes, reinforcing the previously reported FIL safety profile in patients with RA. As the AESIs reported in the long-term safety database with FIL are rare, patients commonly have long exposure times before experiencing an event, which are often associated with end of treatment. As such, EAIRu, EAIR, and EAER are similar.
Reference:
1. Winthrop KL, et al. Ann Rheum Dis 2022;81:184–92
To cite this abstract in AMA style:
Durez P, Feist E, Blanco R, Rajendran V, Verbruggen N, Van Beneden K, Galloway J. The Use of Exposure-Adjusted Event Rates versus Exposure-Adjusted Incidence Rates in Adverse Event Reporting: Insights from Filgotinib Integrated Safety Data in RA [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-use-of-exposure-adjusted-event-rates-versus-exposure-adjusted-incidence-rates-in-adverse-event-reporting-insights-from-filgotinib-integrated-safety-data-in-ra/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-use-of-exposure-adjusted-event-rates-versus-exposure-adjusted-incidence-rates-in-adverse-event-reporting-insights-from-filgotinib-integrated-safety-data-in-ra/