Session Information
Date: Monday, November 14, 2022
Title: SLE – Animal Models Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
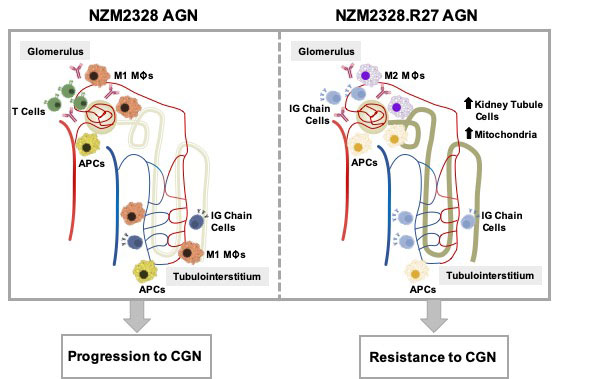
Background/Purpose: Pathologic inflammation is a major driver of kidney damage in lupus nephritis (LN), but the immune mechanisms of disease progression and risk factors for end organ damage are poorly understood. Previous studies established the NZM2328 lupus-prone mouse strain as a model for human proliferative glomerulonephritis (GN). These studies determined that disease in female NZM2328 mice presents in acute (AGN) and chronic (CGN) stages, each of which was associated with genetic loci (Agnz1 and Cgnz1). In addition, male mice and the congenic NZM2328.R27 strain were found to be resistant to the development of chronic nephritis. To characterize molecular profiles through the development of LN, we carried out gene expression analysis of micro-dissected kidneys from lupus-prone NZM2328 mice at different stages of disease severity and examined male and R27 mice as a means to define pathogenic processes associated with disease progression.
Methods: Kidneys from NZM2328 and R27 mice were harvested and the stage of GN was confirmed by histological classification at regular intervals of disease progression. Tissues from young mice, before disease development, were used as a control for diseased mice. Laser capture microdissection was used to isolate glomeruli and tubulointerstitial tissue from control and diseased mice. Total RNA was extracted and hybridized to Affymetrix Mouse Clariom D (NZM2328 and R27 female) or Mouse 430 v2.0 (NZM2328 male) arrays. Differential expression (DE) analysis, gene set variation analysis (GSVA), and linear regression were utilized to define the stages of GN in NZM2328 mice and identify immune populations and processes associated with disease progression.
Results: Gene expression profiling identified a continuum of inflammatory processes associated with progression from acute inflammatory to chronic destructive disease initiated in the glomeruli and progressing to the tubules. Male mice exhibited minimal immune infiltration in the glomeruli resulting in non-progressive renal pathology. Immune infiltrates in the glomeruli of R27 mice expressed a regulatory gene signature and especially a dominance of M2-like macrophages. Moreover, R27 mice manifested an enhanced kidney tubule signature, with evidence of increased mitochondrial and metabolic activity consistent with a functional resistance to cellular damage. The robust tubule signature was associated with the absence of an immune/inflammatory gene signature. Numerous genes in the R27 genetic region were upregulated in NZM2328 nephritic kidneys and could contribute to the protective effect of this interval on the evolution of LN.
Conclusion: Transcriptome analysis revealed distinct immune profiles for AGN, after initial IC deposition in the kidney glomerulus, TGN in which inflammatory cell and pathway enrichment is at its peak, and CGN in which the accumulated insults result in irreversible damage to the kidney tissue. In addition, we identified distinct mechanisms of resistance to chronic disease based on differences in gender and genetics with implications for elucidating risk factors for development of ESRD in human lupus patients.
To cite this abstract in AMA style:
Daamen A, Wang H, Bachali P, Fu S, Grammer A, Lipsky P. The Transcriptomic Landscape of Nephritic Kidneys Reveals Mechanisms for End Organ Resistance to Damage in Lupus-prone Mice [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-transcriptomic-landscape-of-nephritic-kidneys-reveals-mechanisms-for-end-organ-resistance-to-damage-in-lupus-prone-mice/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-transcriptomic-landscape-of-nephritic-kidneys-reveals-mechanisms-for-end-organ-resistance-to-damage-in-lupus-prone-mice/