Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Relapses of ANCA-associated vasculitis (AAV) are associated with death, decreased renal function, and end-stage renal disease. Whether longer-term treatment with glucocorticoids (GC) reduces the risk of relapse is unclear. The objective of this study was to determine the association between dosages of GC and relapse in patients with AAV and update a previous meta-analysis with long-term follow-up and trials with rituximab.
Methods: MEDLINE, EMBASE, Cochrane Clinical Trials, and the Grey Literature were searched from January 1, 2008 until May 26, 2016 without language restriction for studies with patients with AAV. Studies were included if duration of GC use was specified a priori and patients were followed for a minimum of 18 months. Both randomized controlled trials (RCT) and prospective cohort studies were included. Quality of evidence was assessed using modified Newcastle-Ottawa criteria. Meta-analysis was completed using a random-effects model of DerSimonian and Laird.
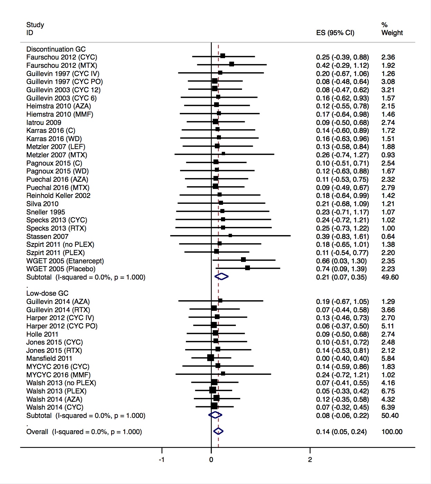
Results: Twenty-four studies met inclusion criteria consisting of 2272 patients. Thirteen (54%) discontinued GC in less than one year. All patients received additional non-GC immunotherapy for induction of remission and 3 studies (<10% of patients) included patients receiving only GC as maintenance therapy. The pooled relapse rate was 14.3 per 100 patient years (95% CI 4.5, 24.0). Relapse was more frequent when discontinuation of GC at any time was compared to long-term, low-dose GC (20.7 per 100 patient years, 95% CI 6.8,34.5 as compared to 8.0 per 100 patient years, 95% CI 5.7,21.7). Multivariable linear meta-regression confirmed that long-term, low-dose GC was associated with lower relapse rates (b = -0.16, 95% CI -0.26,0.07, P = 0.001) and overall follow up time was associated with increased relapse rates (b = 0.003, 95% CI 0.001,0.006, P = 0.002). Time to discontinuation of non-GC immunotherapy, time to GC discontinuation, study type, renal function, presence of relapsing disease at baseline, and immunotherapy that included oral cyclophosphamide did not demonstrate any significant association with relapse rates.
Conclusion: Long-term, low-dose GC is associated with decreased relapse rates in patients with AAV. The clinical severity and consequences of the excess relapses are not well studied. Characterization and reporting of adverse events limited analysis. These data have implications for both clinical care and trial design. RCTs are needed to determine optimal GC administration in patients with AAV.
Figure Legend: Random effects meta-analysis of long-term, low dose GC as compared to GC discontinuation on relapse per patient year. CYC = cyclophosphamide, MTX = methotrexate, IV = intravenous, PO = oral, MMF = mycophenolate mofetil, AZA = azathioprine, LEF = leflunomide, RTX = rituximab, PLEX = plasma exchange, C = continuation arm, WD = withdrawal arm.
To cite this abstract in AMA style:
Rodrigues J, Collister D, Archer A, Cheema K, Alexander P, Pagnoux C, Thabane L, Merkel PA, Jayne D, Walsh M. The Steroid Tapering in ANCA Vasculitis Evaluation Study (STAVE) 2: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-steroid-tapering-in-anca-vasculitis-evaluation-study-stave-2-a-systematic-review-and-meta-analysis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-steroid-tapering-in-anca-vasculitis-evaluation-study-stave-2-a-systematic-review-and-meta-analysis/