Session Information
Date: Monday, November 6, 2017
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment III: Biomarkers
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: We have developed the iCHIP1,2 to profile repertoires of serum autoantibodies. The first iCHIP application was the SLE-key RuleOut test3,4 , to rule out a diagnosis of SLE with 94% sensitivity, 75% specificity, and 93% negative predictive value (NPV). In current study we asked whether the SLE-key test results are stable over time, and whether the test results can be used as a serologic signature of lupus which is independent of treatment and/or disease activity.
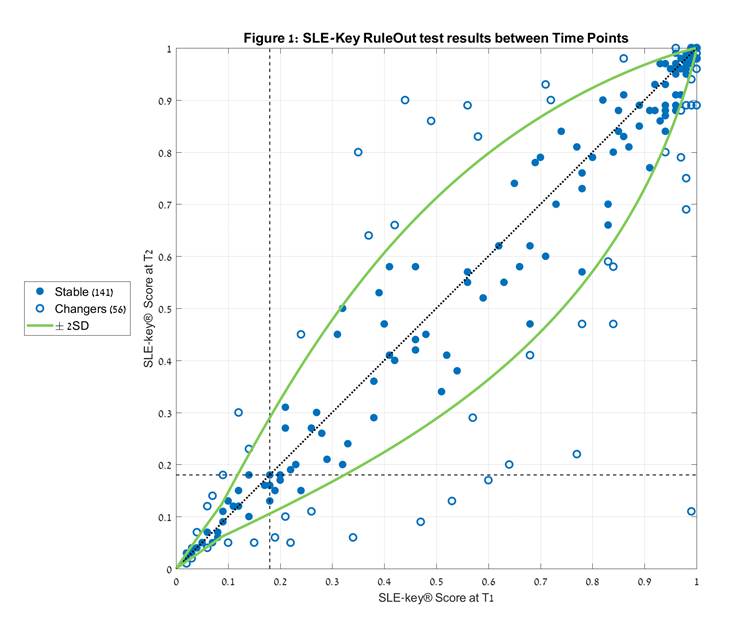
Methods: We determined the SLE-key scores for 197 paired serum samples submitted by 4 independent academic lupus centers. SLEDAI scores at the time of blood draw ranged from 0 to 22. The mean absolute SLEDAI difference between the paired samples was 6.2±3.4; 60% of pairs manifested a decrease in SLEDAI score at time point 2 (T2) relative to time point 1 (T1), and 40% showed an increase. T2-T1 time interval ranged from several weeks to 12 years (mean 1.5 ± 2.3 years). The sensitivity of the SLE-key score between T1 and T2 to technical variability was determined independently. Subjects exhibiting score changes of more than 2SD were defined as “changers”, and within 2SD were classified as “stable” (Figure 1).
Results: The SLE-Key test identifies an SLE-specific serologic signature based on a profile of IgG and IgM autoantibodies to a combination of two nucleic acids (complex ssDNA and a defined oligonucleotide) and three nuclear antigens (u1snRNP, Smith, and histone). This signature was found to be stable (within 95% confidence) in 71.6% (141/197) of subjects. Moreover, 84% (47/56) of the “changers” had no resultant change in their SLE-key RuleOut status (i.e. “Ruled Out” or “Not Ruled Out”). Of the remaining 9 patients, 7 changed from Not Ruled Out at T1 to Ruled Out at T2, and 2 subjects changed from SLE Ruled Out at T1 to Not Ruled Out at T2. The stability of the signature was independent of age, race, and time post diagnosis or between sampling. Furthermore, the signature was also independent of SLEDAI score.
Conclusion: The SLE-key RuleOut test detects a serologic signature which remains stable, irrespective of time or SLEDAI in >70% of subjects. This signature may therefore be helpful clinically to confirm a historical diagnosis of SLE in patients that currently have low disease activity. Even among the “changers”, the large majority had no change in their SLE-key RuleOut status. The stability of this signature over time indicates that it functions as a biomarker of SLE, but may not reflect disease activity. We are currently studying the possible reasons for a changing score in the remaining 30% of subjects, and the differences between the “changers” and the “stable” subjects.
References
1 Fattal et al; Immunology 2010 2Cohen IR, LOA 2016 3Putterman et al; J Immunol Methods, 2016 4Massenburg et al; LOA 2017
Acknowledgements: authors wish to acknowledge Cohen-Gindi O, Lerner M, Tarnapolski O, Blumenstein Y, Javaherian A, Pitts J, Barton M and Wong E
To cite this abstract in AMA style:
Putterman C, Petri M, Caricchio R, Oates JC, Safer P, Jakobi-Brook K, Sorek R, Gluzman I, Wallace S, Cohen IR. The SLE-Key Test Detects an SLE Serologic Signature That Persists over Time and Is Independent of Disease Activity [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-sle-key-test-detects-an-sle-serologic-signature-that-persists-over-time-and-is-independent-of-disease-activity/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-sle-key-test-detects-an-sle-serologic-signature-that-persists-over-time-and-is-independent-of-disease-activity/