Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA) is an oral reversible JAK inhibitor engineered for greater selectivity for JAK1 versus JAK2, JAK3, and TYK2, and is currently being assessed for the treatment of RA. Here we report on an integrated, long-term safety analysis of UPA in Japanese patients (pts) with RA from the UPA clinical development program.
Methods: Japanese pts were included from three studies: the Phase 2b/3 SELECT-SUNRISE study1 (which only enrolled Japanese pts), and the Phase 3 SELECT-EARLY2 and SELECT-MONOTHERAPY3 studies (which included subsets of Japanese pts). In SELECT-SUNRISE, pts with an inadequate response (IR) to csDMARDs were randomized to receive UPA 7.5 mg, 15 mg, or 30 mg once daily (QD), or matching placebo (PBO), for 12 weeks in addition to background csDMARDs. In SELECT-EARLY, MTX-naïve pts received monotherapy with UPA 7.5 mg (Japan only), 15 mg, or 30 mg QD, or MTX, for 24 weeks. In SELECT-MONOTHERAPY, MTX-IR pts received monotherapy with UPA 15 mg or 30 mg QD, or continued with MTX monotherapy, for 14 weeks. Each study included the option to continue into a long-term extension. The exposure adjusted event rates (EAERs) of adverse events (AEs) and AEs of special interest (AESI) per 100 pt-years (PY) are reported. Results in Japanese pts were compared with the global population in UPA Phase 3 studies.
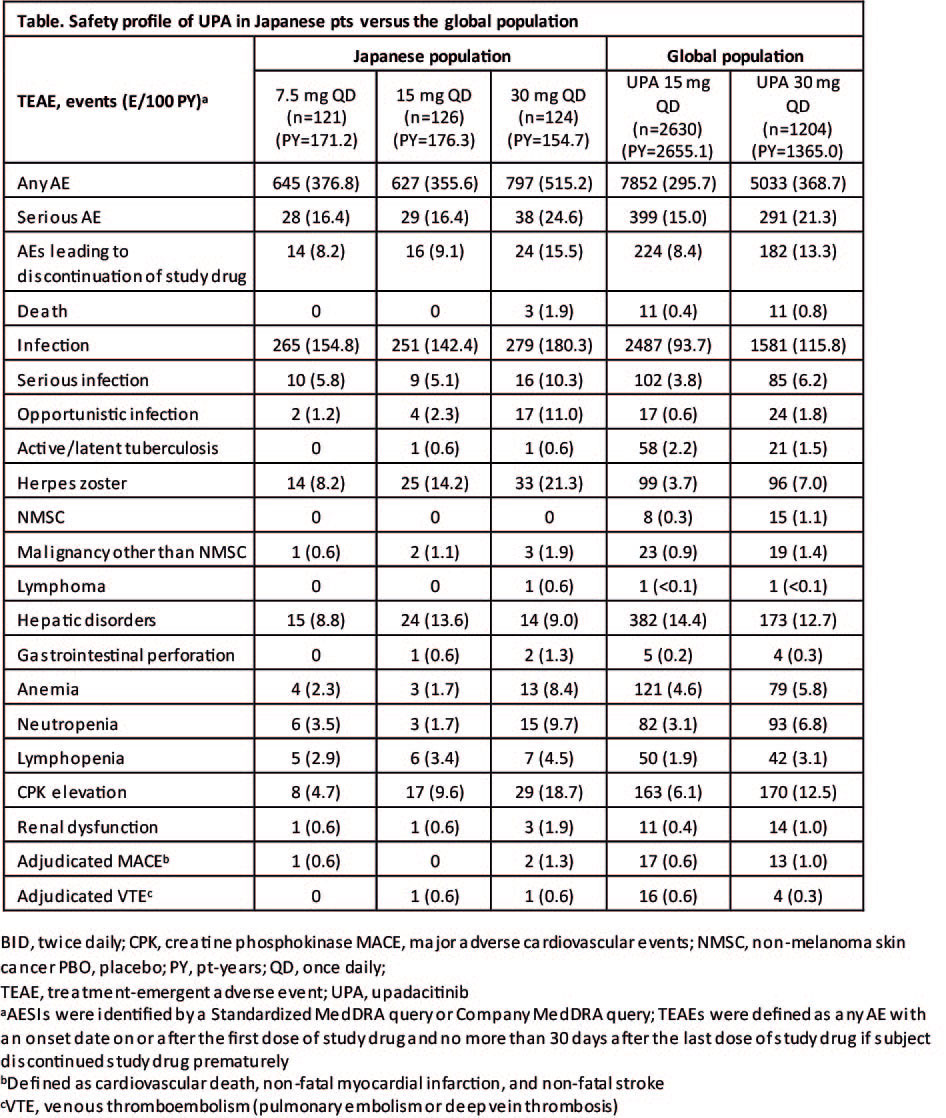
Results: Japanese pts (n=371) received UPA 7.5 mg QD (n=121), 15 mg QD (n=126), or 30 mg QD (n=124). Exposure to UPA was 171.2, 176.3, and 154.7 PY in the 7.5 mg, 15 mg, and 30 mg groups, respectively, with mean exposures of 516.8, 511.0, and 455.5 days. The demographic characteristics of pts in all the treatment groups were generally well balanced; the majority of pts were female and aged between 40 and 64 years. Among the Japanese population, EAERs were consistently higher in the 30 mg group compared with the 15 mg and 7.5 mg groups (Table). EAERs in the 15 mg and 7.5 mg groups were comparable. In comparison with the global population, the EAER in the 15 mg and 30 mg groups was higher in the Japanese population. The EAER of infections (including herpes zoster) was also higher in the Japanese population compared with the global population. The EAERs of AESI (including malignancies, cardiovascular disorders, hepatic disorders, and laboratory abnormalities) were comparable between the Japanese and global populations.
Conclusion: No new safety risks were identified with administration of UPA in Japanese pts. As reported with other JAK inhibitors, herpes zoster occurred at higher rates on UPA in Japanese pts versus the global population. The 7.5 mg and 15 mg doses had lower EAERs of overall AEs, infections and laboratory-related AEs compared to that observed with 30 mg.
References
- https://clinicaltrials.gov/ct2/show/NCT02720523
- van Vollenhoven R, et al. Arthritis Rheumatol 2018;70(Suppl 10): Abstract 891
- Smolen JS, et al. Arthritis Rheumatol 2018;70(Suppl 10): Abstract 889
To cite this abstract in AMA style:
Yamaoka K, Tanaka Y, Kameda H, Hendrickson B, Meerwein S, Zhang Y, Takeuchi T. The Safety Profile of Upadacitinib in Japanese Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-safety-profile-of-upadacitinib-in-japanese-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-safety-profile-of-upadacitinib-in-japanese-patients-with-rheumatoid-arthritis/