Session Information
Date: Sunday, November 12, 2023
Title: (0380–0422) RA – Diagnosis, Manifestations, and Outcomes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: To investigate, in primary care, whether testing anti-CCP3 antibodies in anti-CCP2 negative individuals with musculoskeletal (MSK) symptoms, improved the prediction of inflammatory arthritis (IA)/rheumatoid arthritis (RA) progression.
Methods: A total of 469 anti-CCP2 negative individuals who presented to their general practitioner (GP) with new MSK symptoms were included in this study. All participants underwent baseline anti-CCP3 testing (QUANTA Lite CCP3; Inova Diagnostics) and received a questionnaire 12 months after enrolment assessing their disease status. The GPs of those individuals who reported progression to IA/RA were contacted by a rheumatologist to confirm the diagnosis. Univariate and multi-variate analyses were performed to establish variables associated with disease progression.
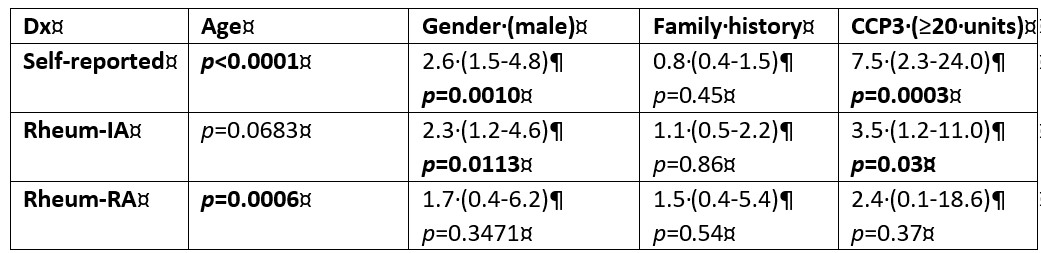
Results: Both the progression rate towards IA/RA and the prevalence of anti-CCP3 antibodies in anti-CCP2 negative individuals with MSK symptoms were low. Only 61/469 (13.0%) participants reported disease progression of which 43/61 (70.5%) and 13/61 (21.3%) were confirmed to have a diagnosis of IA and RA, respectively. Anti-CCP3 was positive in only 16/469 (3.4%) anti-CCP2 negative individuals. However, interestingly, in univariate analysis, anti-CCP3 positivity was associated with self-reported progression (p< 0.001) and with a diagnosis of IA (p=0.03), but not with a diagnosis of RA (p=0.37). In contrast, when considering antibody levels, anti-CCP3 differed significantly between progressors and non-progressors (p< 0.0001) for all three categories (self-reported progression, IA and RA diagnosis).
At the manufacturer’s cut-off (≥20 units) the sensitivity for progression to IA/RA ranged from 8.0-14.0% with high specificity (≥97.0%). The corresponding odds ratios (OR) ranged from 2.4 (95% CI 0.3-20.0) to 7.8 (95% CI 2.8-21.8). Interestingly, when cut-offs were optimized for F-1 score, lower cut-off values (5 units) significantly increased the OR for progression in all three categories. After correcting for confounding factors (age, gender), in multi-variate analysis anti-CCP3 levels remained significantly associated with diagnosis of RA (p=0.02).
Conclusion: The rate of progression to IA/RA in anti-CCP2 negative individuals with MSK symptoms seen in primary care setting was low over a 12-months follow-up period. Our results showed that anti-CCP3 antibody levels have a potential role in improving prediction in IA/RA progression in anti-CCP2 negative individuals with MSK symptoms. Future studies are warranted to validate the cut-off values for anti-CCP3 antibodies with best prediction accuracy in this population.
To cite this abstract in AMA style:
Di Matteo A, Mankia K, Garcia-Montoya L, Nam J, sharrack S, Mahler M, Emery P. The Role of anti-CCP3 Antibodies in anti-CCP2 Antibody Negative Patients with Musculoskeletal Symptoms [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-role-of-anti-ccp3-antibodies-in-anti-ccp2-antibody-negative-patients-with-musculoskeletal-symptoms/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-role-of-anti-ccp3-antibodies-in-anti-ccp2-antibody-negative-patients-with-musculoskeletal-symptoms/